All published articles of this journal are available on ScienceDirect.
Experimental Study of CO2 Absorption in Potassium Carbonate Solution Promoted by Triethylenetetramine
Abstract
Background:
Separation of CO 2 as the major cause of global warming is essential. In this work, potassium carbonate (K 2 CO 3 ) solution was selected as a base solvent for CO 2 absorption due to its ease of regeneration energy, low cost and low environmental impact. However, the absorption rate of CO 2 with K 2 CO 3 needs to be improved by adding a suitable promoter. Therefore, the performance of CO 2 in K 2 CO 3 solution promoted by triethylenetetramine (TETA) in terms of absorption capacity and absorption rate of CO2 was studied.
Method:
Experiments were conducted at a total concentration of 2.5 (M) with different TETA mole fractions at temperatures of 303, 313 and 323 K, and CO2 partial pressure up to 30 kPa using a stirred cell reactor. The effect of CO2 partial pressure, temperature and concentration of TETA on absorption capacity and absorption rate of CO2 in K2CO3+TETA solution was discussed in detail.
Results:
The CO2 loading capacity obtained in this work was compared with monoethanolamine (MEA) and a better performance was observed for K2CO3+TETA solution. In addition, experimental results revealed that the addition of TETA to K2CO3 improved the CO2 reaction rate. Finally, the response surface methodology was employed to correlate the CO2 solubility. It was found that the correlated data are in good agreement with the experiment results.
Conclusion:
As an overall conclusion, the solution of K2CO3+TETA can be used as a promising absorbent in post combustion CO2 capture processes.
1. INTRODUCTION
The fossil fuel power plants are considered as the main source of greenhouse gases emission, which increase CO2 concentration in the atmosphere and environmental issues [1, 2]. In order to reduce the CO2 emission, it is essential to capture CO2 from fossil fuel power plants [3]. Several technologies such as oxy-fuel combustion, pre combustion and post-combustion are used for CO2 capture [4]. Among them, post-combustion using a solvent is widely employed as one of the most reliable and economical methods for CO2 capture [5]. One of the main challenges of this method is the selection of a suitable solvent [6]. To be selected as a solvent, it needs to satisfy several desired properties, including high CO2 loading capacity, low volatility, high absorption rate, high thermal stability and low heat of absorption [7]. Different types of solvents have been studied for the CO2 capture such as ionic liquids, amino acid salts, inorganic solvents and alkanolamines [8]. The ionic liquids are used as solvents because of some advantages such as high CO2 capacity and thermal stability. However, they have disadvantages such as high cost and viscosity [9, 10]. Amino acid salt solutions gained interest as a solvent because of low volatility, less toxicity and low thermal stability. The main challenges associated with amino acid salts are high cost and precipitation of carbonates at high concentration [11, 12]. The inorganic solvents such as tri-sodium phosphate and potassium carbonate are another category of solvents with desired characteristics such as low corrosion rate and lower heat of absorption than amines [13]. Alkanolamines such as monoethanolamine (MEA) have` been used extensively in many CO2 capture processes because of its low solvent cost and fast reaction kinetic [14]. However, MEA has several disadvantages such as low CO2 absorption capacity, thermal or chemical degradation, high regeneration energy requirement and corrosive [15]. Many researchers have tried to find a suitable solvent that minimize the cost and penalty in the power plant efficiency [16]. The solution of potassium carbonate (K2CO3) has been received much attention due to its advantages such as low environmental impact, low solvent cost, low absorption heat and low thermal degradation [17,18]. However, its slow reactivity with CO2 needs to be improved [19]. To solve this problem, researchers have suggested the addition of promoters to K2CO3 in order to enhance the absorption rate. As an example, Cullinane and Rochelle [20], reported the absorption rate of CO2 in 20-30 wt% potassium carbonate promoted by 0.6 kmol/m3 piperazine (PZ) at temperatures from 313 to 353 K. They concluded that the CO2 absorption rate was increased by the addition of PZ. Kim et al. [21] investigated the absorption rate of CO2 in blend solution of 15 wt% potassium carbonate and 7.5 wt% homopiperazine (homoPZ). They observed that K2CO3+homoPZ has a higher absorption rate than pure K2CO3. Thee et al. [22] used 5 and 10 wt% monoethanolamine (MEA) as a promoter for K2CO3. The results revealed that CO2 absorption rate in K2CO3+MEA solution is higher than potassium carbonate. Thee et al. [23] studied the effect of the addition of boric acid to 30 wt% potassium carbonate at 353.15 K. It was found that boric acid has a positive effect on CO2 absorption rate at blended solution. Shen et al. [24] examined reaction kinetic in a mixture of 35 wt% K2CO3 and arginine at temperatures from 313 K to 343 K. It was discovered that arginine can be an effective promoter for potassium carbonate. Thee et al. [25] selected three amino acids, including glycine, proline and sarcosine as potential promoters for potassium carbonate solution and obtained reaction kinetic at temperatures from 313.15 to 353.15 K. Kim et al. [26] evaluated CO2 absorption rate and capacity of potassium carbonate solution promoted by 2-methylpiperazine at 313, 333 and 353 K. They found that the addition of a small amount of 2-methylpiperazine to potassium carbonate can increase the CO2 absorption capacity and rate. Bhosale et al. [27] added ethylaminoethanol to potassium carbonate and calculated reaction kinetics at temperatures (303-318 K). It was found that ethylaminoethanol acts as a good promoter. This motivated us to select triethylenetetramine (TETA) as a promoter in order to improve absorption rate of CO2 in K2CO3 solution. TETA with four functional groups, including two primary and two secondary amino groups has advantages compared to other promoters like methyldiethanolamine, 2-((2-aminoethyl)amino)ethanol, diethanolamine, 2-amino-2-methyl-1-propanol and monoethanolamine in terms of fast reaction kinetic, high CO2 solubility and low regeneration energy. Due to these advantages, TETA was chosen as a promoter for potassium carbonate. Therefore, in this work, absorption capacity and rate of absorption of CO2 in K2CO3+TETA were evaluated using a stirred cell reactor at a temperature range of (303.15 to 323.15) K and pressure up to 30 kPa. The CO2 absorption capacity of K2CO3+TETA as a function of pressure, temperature and concentration was also correlated by response surface methodology. In fact, this work presents new CO2 solubility data in K2CO3+TETA, which are important and necessary for modeling of CO2 absorption processes in industrial application.
2. REACTION MECHANISM
When CO2 is absorbed in potassium carbonate solution, the following reactions take place [26, 28]:
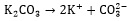 |
(1) |
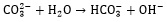 |
(2) |
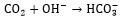 |
(3) |
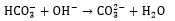 |
(4) |
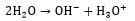 |
(5) |
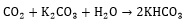 |
(6) |
In comparison to the potassium carbonate solution, alkanolamines have a high rate of reaction with dissolved CO2. The general reaction mechanism between CO2 and amine can be indicated in Eqs. (7, 8), which R1 and R2 are carbon side chains [29]:
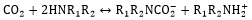 |
(7) |
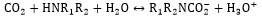 |
(8) |
3. MATERIALS AND METHODS
3.1. Materials
All chemicals used in this study, including potassium carbonate (K 2 CO 3 ), triethylenetetramine (TETA) and monoethanolamine (MEA) with grade >98% pure were purchased from Acros Organics and used without any further purification. The chemical structure of TETA is given in Fig. (1). Experiments were carried out in blended solutions of K2CO3 and TETA with the compositions of 2.25 M K2CO3 + 0.25 M TETA, 2 M K2CO3 + 0.5 M TETA, 1.75 M K2CO3 + 0.75 M TETA, 1.5 M K2CO3 + 1 M TETA in temperatures 303-323 K and pressures up to 30 kPa.
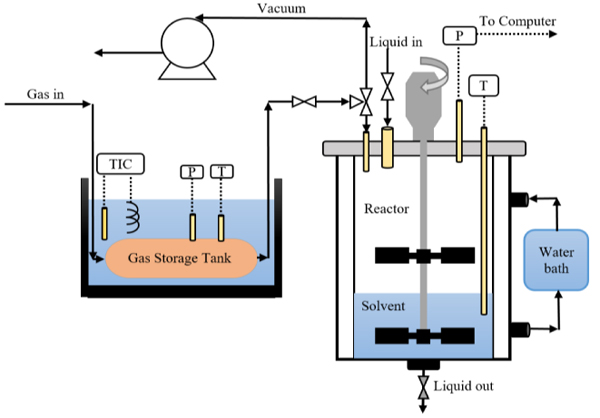
3.2. Equipment and Experimental Procedure
The CO2 absorption measurement procedure and equipment used in this study are same as employed in our previous works [30-33]. A glass stirred cell reactor was used to measure CO2 solubility and initial absorption rate of CO2 in the solution of K2CO3 promoted by TETA, as shown in Fig. (2). This reactor is connected to a gas storage tank and a water bath to control the temperature during the experiments. Both stirred cell reactor and gas storage tank are connected with temperature sensor and pressure transmitter. Before running the experiment, the reactor was purged with a vacuum pump. The solvent was placed in the reactor and the solution is allowed to reach a desired temperature. After that, CO2 was fed to gas storage tank from gas cylinder and was heated using a water bath. Next, CO2 was transferred to reactor from gas storage tank, and stirrer was switched on. The total moles of CO2 transferred can be calculated from:
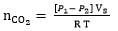 |
(9) |
Where VS, P1 and P2 are the volume, initial and final pressure of CO 2 in the gas storage tank, respectively. The CO2 partial pressure in the reactor is decreased over time because of the chemical absorption of the CO2 in solution and the pressure drop in the reactor is monitored and recorded every second by a pressure transmitter. Therefore, the initial absorption rate can be obtained from slope of CO2 partial pressure changes versus time. The equilibrium partial pressure of CO2 and the moles of CO2 remaining in equilibrium cell were obtained by Eqs. 10 and 11, respectively:
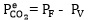 |
(10) |
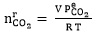 |
(11) |
Absorption capacity of CO2 in the solution of K2CO3+TETA is defined in terms of CO2 loading α (mole CO2 absorbed per mole of solvent).
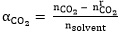 |
(12) |
As mentioned earlier, the method of CO2 loading calculation can be found in our previous works. Three repeat runs were taken to check the reproducibility and data presented here represent averages.
4. RESULTS AND DISCUSSION
4.1. CO2 Absorption Capacity in K2CO3+TETA Solution
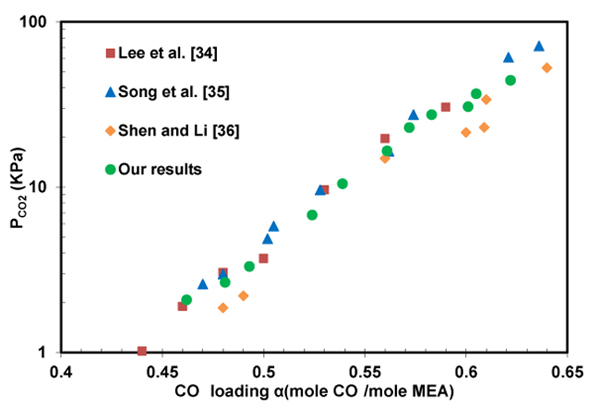
In order to validate equipment and experimental procedure, the loading capacity of CO2 in the solution of 2.5 M monoethanolamine was measured at 313 K. Fig. (3) shows a good agreement exists between the CO2 absorption capacities measured in this work and the values previously reported [34-36].
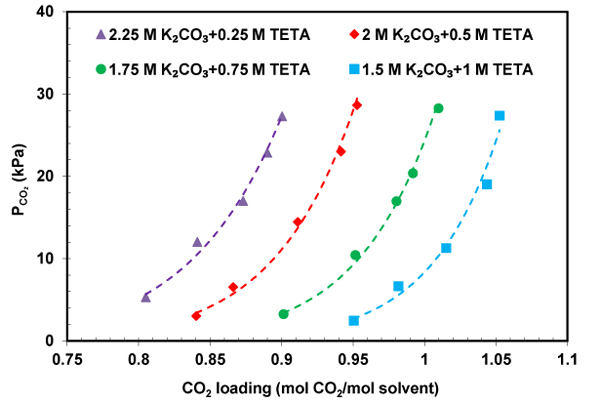
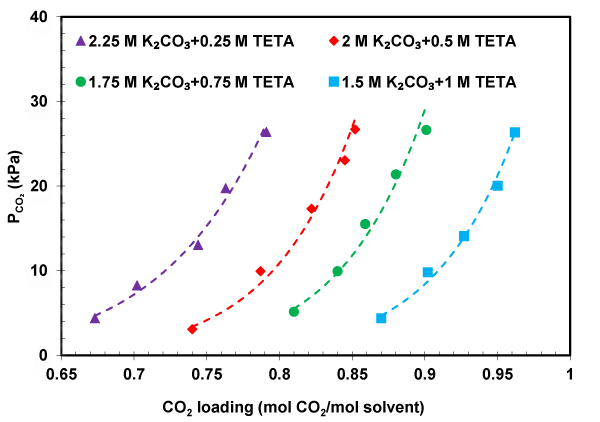
CO 2 solubility data presents useful information for industrial application in design and modeling of CO 2 absorption in CO 2 capture process [24]. In this work, the CO 2 solubility in K 2 CO 3 +TETA solution was measured at temperatures 303-323 K, CO 2 partial pressures up to 30 kPa, and results listed in Table 1 . As can be observed in Table 1 , temperature has a negative effect on the CO 2 loading. In the other words, an increase in the temperature from 303 to 323 K leads to a decrease in loading capacity from approximately 0.8 to 0.65. This reduction can be explained by the fact that the equilibrium would shift in the backward direction with increasing temperature. In order to study the effect of pressure and concentration on the performance absorption capacity of the solvent, CO 2 partial pressure as a function of CO 2 solubility was plotted in Figs. (4-6) . It can be seen that K 2 CO 3 +TETA solution absorbs more CO 2 at higher pressure which can be due to the increase in the driving force from the gas phase to the interface. According to Figs. (4-6) , the absorption capacity also increases with the increase in concentrations of TETA. The highest CO 2 solubility was achieved at TETA mole fraction equal to 0.4. The reason for this behavior could be explained by the fact that TETA as a polyamine has four amine groups Fig. (1) that means TETA can absorb more CO 2 . Thus, a smaller amount of solvent is needed for CO 2 absorption with high efficiency in absorber that leads to a lower cost. The absorption performance of K 2 CO 3 +TETA solution was compared with pure K 2 CO 3 , at 313.15 K, shown in Fig. (5) . It is clear that CO 2 loading of K 2 CO 3 +TETA solution is much higher than pure K 2 CO 3 in the order: 1.5 M K 2 CO 3 + 1 M TETA > 1.75 M K 2 CO 3 + 0.75 M TETA > 2 M K 2 CO 3 + 0.5 M TETA > 2.25 M K 2 CO 3 + 0.25 TETA > 2.5 M K 2 CO 3 suggesting high ability of TETA for CO 2 capture.


| 2.25 M K2CO3 + 0.25 M TETA | 2 M K2CO3 + 0.5 M TETA | 1.75 M K2CO3 + 0.75 M TETA | 1.5 M K2CO3 + 1 M TETA | ||||
|---|---|---|---|---|---|---|---|
| PCO2 (kPa) | α | PCO2 (kPa) | α | PCO2 (kPa) | α | PCO2 (kPa) | α |
| T = 303.15 K | |||||||
| 05.31 | 0.805 | 03.05 | 0.840 | 03.27 | 0.901 | 02.48 | 0.950 |
| 12.04 | 0.841 | 06.55 | 0.866 | 10.44 | 0.951 | 06.65 | 0.981 |
| 17.03 | 0.873 | 14.47 | 0.911 | 16.99 | 0.980 | 11.28 | 1.015 |
| 22.88 | 0.890 | 23.02 | 0.941 | 20.37 | 0.991 | 19.03 | 1.043 |
| 27.29 | 0.901 | 28.67 | 0.952 | 28.26 | 1.009 | 27.37 | 1.052 |
| T = 313.15 K | |||||||
| 04.95 | 0.739 | 04.83 | 0.801 | 05.19 | 0.863 | 03.35 | 0.898 |
| 09.44 | 0.769 | 10.93 | 0.853 | 08.21 | 0.890 | 07.02 | 0.934 |
| 14.27 | 0.793 | 16.05 | 0.870 | 13.04 | 0.908 | 15.99 | 0.978 |
| 21.32 | 0.818 | 20.85 | 0.889 | 19.27 | 0.933 | 22.52 | 1.001 |
| 28.44 | 0.842 | 27.33 | 0.910 | 26.25 | 0.961 | 28.04 | 1.011 |
| T = 323.15 K | |||||||
| 04.37 | 0.673 | 03.08 | 0.740 | 05.14 | 0.810 | 04.37 | 0.870 |
| 08.26 | 0.702 | 09.94 | 0.787 | 09.95 | 0.840 | 09.82 | 0.902 |
| 13.05 | 0.744 | 17.33 | 0.822 | 15.51 | 0.859 | 14.09 | 0.927 |
| 19.74 | 0.763 | 23.05 | 0.845 | 21.37 | 0.882 | 20.03 | 0.951 |
| 26.38 | 0.791 | 26.69 | 0.852 | 26.62 | 0.901 | 26.35 | 0.962 |
In Fig. (7), the solubility of CO2 in K2CO3+TETA solution measured in this work was compared with the solubility of CO2 in some solvents at 313.15 K. It can be found that the blend of K2CO3+TETA has the highest CO2 loading when compared to MEA and other solvents. This result confirms the high performance of this solvent for CO2 capture.
4.2. Correlation of Experimental Data
Response surface methodology (RSM) is an efficient method to obtain the sensitivity analysis of a process and optimum condition of a multivariable system [47]. In addition, RSM can be applied for investigation of effect of factors on response, and the relationship between response variables [48]. In this work, the response surface methodology was used to correlate the CO2 solubility experimental data (α) as a function of temperature (T), pressure (PCO2) and TETA mole fraction (R) in the solution of potassium carbonate promoted by TETA. Three parameters including pressure, promoter mole fraction and temperature were selected as independent variables, and the validity of the model was evaluated using analysis of variance ANOVA on response parameter (CO2 solubility). The effect of these parameters on the CO2 solubility was studied. The results showed that mole fraction of TETA has the highest impact on CO2 solubility, while the interaction of pressure and temperaturewas found to be the least important parameter. In order to check the accuracy of the obtained model, F-value, R-squared and adjusted R-squared were determined using ANOVA analysis. The R-squared and adjusted R-squared for this system have been found to be 0.986 and 0.981, respectively. These high values of R2 and R2adj show the significance of model terms. The F-value of the reduced quadratic model was also found to be 85.42 which indicates the validity of model.

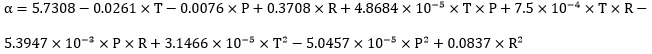 |
(13) |
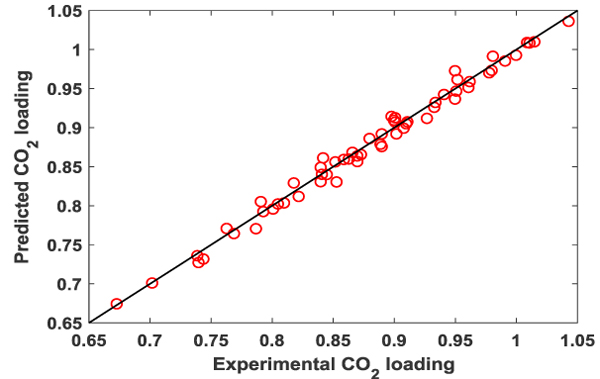
The predicted CO2 absorption capacity by eq. (13) were plotted against experimental data obtained in this work in Fig. (8). It can be clearly seen that the good agreement exists between the values calculated by the eq. (13) for the absorption capacity of CO2 and the experimental results with the average absolute deviation percent (AAD) equal to 1.19%.
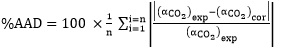 |
(14) |
4.3. CO2 Absorption Rate in K2CO3+TETA Solution
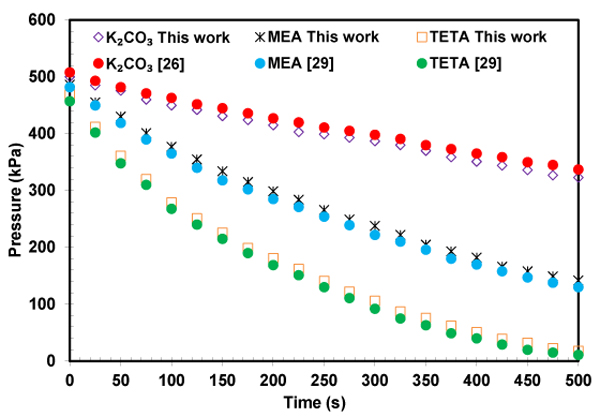
As previously mentioned, the main challenge of potassium carbonate is its slow rate with carbon dioxide, which leads to the larger absorber size. In this work, TETA was added as a promoter to potassium carbonate in order to enhance the rate of absorption. To check the setup and experimental method, the pressure decay during CO2 absorption in solutions of pure MEA, TETA and K2CO3 at 313.15 K 313.15 K was presented in Fig. (9) and comparedwith literature data [26, 29]. A good agreement was found between results obtained in this work and the literature. As expected, the absorption rate in TETA solution is much higher than MEA and K2CO3 because of its structure feature.
The profile of CO2 partial pressure versus time for the solution of K2CO3+TETA at different concentrations and temperatures were illustrated in Figs. (10-12) to investigate the effect of the addition of TETA to potassium carbonate on the absorption rate. The initial slope of the pressure curves over time is an indication of CO2 absorption rate. It was found that the CO2 partial pressure decreases with increasing time until a gas-liquid equilibrium is reached. The time required to obtain an equilibrium state is different for each solutions. This pressure reduction in the equilibrium cell shows the amount of gas absorption. According to Figs. (10-12), the addition of small amount of TETA to potassium carbonate has been led to a significant increase in the absorption rate that is favorable for the CO2 capture process. The structure feature of TETA with two primary amino groups and two secondary amino sites, and also an increase in the rate of reaction of CO2 with OH- due to increase of PH of solution can be applied to explain this rate enhancement. It also can be observed from Fig. (11) that the mixed absorbent has higher absorption rate compared to single potassium carbonate. This result proves the slow kinetics between potassium carbonate and CO2.
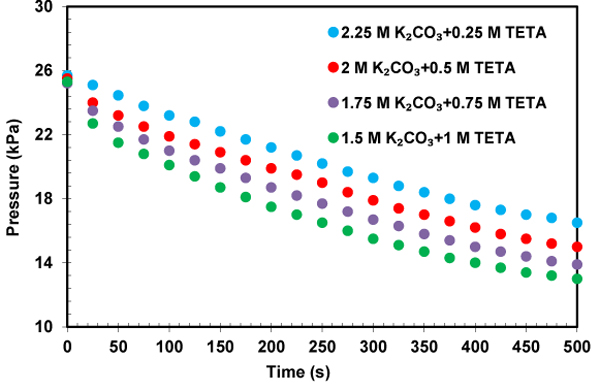

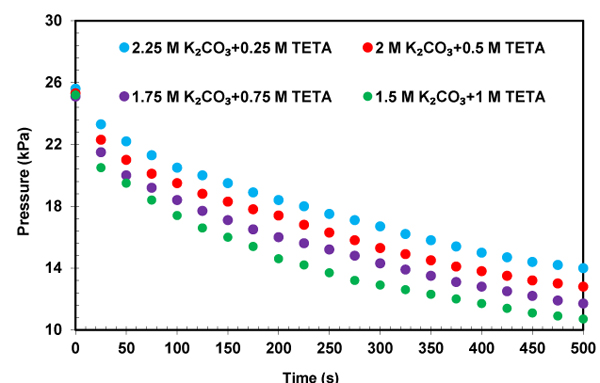
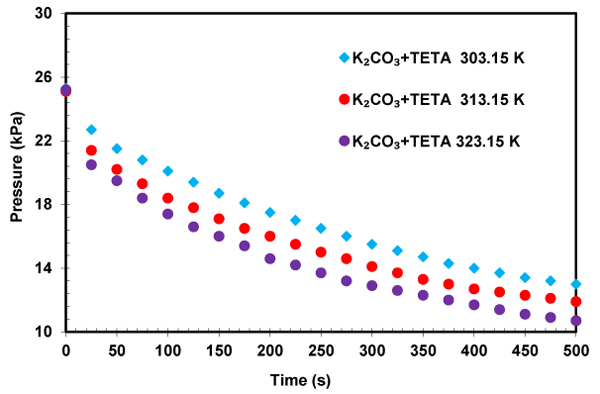
The influence of the temperature on the initial absorption rate in solution of K2CO3+TETA was investigated and shown in Fig. (13). As expected, higher temperature resulted in an increase in reactivity of CO2 with solution of K2CO3+TETA. The obtained results shows that TETA has a good ability as a promoter for potassium carbonate and TETA+K2CO3 solution could be an appropriate solvent for CO2 capture because of its high absorption rate and capacity.
CONCLUSION
In this work, CO 2 solubility and initial absorption rate in K 2 CO 3 +TETA solution were investigated at temperatures 303-323 K and the partial pressures up to 30 kPa using a stirred cell reactor. Obtained results revealed that CO2 absorption capacity of K2CO3+TETA solution increase with the increase in TETA mole fraction, and decreased with the increase in temperature. The results also indicated that the K2CO3+TETA solution has absorption capacity higher than MEA. A correlation was presented to predict CO2 loading capacity in solution, and predicted values were found in excellent agreement with the experimental results. Therefore, considering these results, K2CO3+TETA solution can be used as an alternative solvent to MEA. However, in order to make better judgment about this solvent, more investigation needs to be performed to determine the corrosion rate and absorption heat of K2CO3+TETA solution.
NOMENCLATURE
| AEEA | = 2-((2-aminoethyl)amino)ethanol |
| MDEA | = Methyldiethanolamine |
| K-lys | = potassium lysinate |
| K2CO3 | = Potassium carbonate |
| HomoPZ | = Homopiperazine |
| PZEA | = 2-(1-piperazinyl)-ethylamine |
| DEA | = Diethanolamine |
| K-Ser | = Potassium serinate |
| TETA | = Triethylenetetramine |
| AMP | = 2-Amino-2-methyl-1-propanol |
| DETA | = Diethylenetriamine |
| MEA | = Monoethanolamine |
| PZ | = Piperazine |
| SG | = Sodium glycinate |
| PV | = Vapor pressure of solution |
| PeCO2 | = The equilibrium partial pressure of CO 2 |
| n solvent | = The moles of solvent in liquid phase |
| P F | = Final pressure of reactor |
| α: | = CO2 solubility (mole CO2/mole of absorbent) |
| V | = V Volume of reactor |
| V s | = Volume of gas storage tank |
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author (editor) declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Decleared none.


