All published articles of this journal are available on ScienceDirect.
The Physicochemical Analysis of the Interaction Reaction of Potassium Chloride with Ammonium Hydrosulfate
Abstract
Introduction
This paper represents the results of exploratory research on the production of ammonium-potassium sulfate salt and by-products. Information on the use of the results of fundamental research for the creation of new technological processes is provided. The increase in the range of products based on ammonium salts is a priority task for the industrial enterprise “KazAzot” JSC (Kazakhstan, Aktau). Another task is to expand the areas of application of products with derivatives based on ammonium hydrosulfate. Furthermore, combined fertilizers can be included in the list of products. The aim of the work is to scientifically substantiate physicochemical transformations in reaction mixtures, such as obtaining ammonium-potassium sulfate-fertilizer with three target components.
Methods
The experimental work was conducted in a laboratory shaft electric furnace, with observations of changes in the reaction mass and determination of the pH of the gas phase. The produced products were subjected to an X-ray phase analysis on the diffractometer Miflex 600 (Japan) with CuKɑ. In addition, the physicochemical changes of reaction mixtures and synthesized salts were studied with the help of the derivatograph of the Q-1000/D system by “MOM” F. Paulik, J. Paulik, L. Erdey and LabSys Evo TG-DTA/DTG (Setaram, France), NETZSCH STA 443 F3 in the nitrogen atmosphere. The thermodynamic evaluation of the probability of reactions was obtained using the Astra-4 program complex HCS-5.1 Chemistry (Outokumpu).
Results
It has been established that in the temperature range 157 - 260 °C at equal molar ratios of NH4HSO4÷KSO4 the condensed product of the reaction is a double salt ammonium-potassium sulfate (NH4KSO4) and its decomposition into constituent sulfates occurs above 260 °C with simultaneous pyrolysis of ammonium sulfate to complete formation of ammonium hydrosulfate and ammonia. At incomplete pyrolysis of ammonium sulfate in the cooled residue is present (NH4)3HS2O8, so salt formed from 2 ammonium sulfates: (NH4)2SO4∙NH4HSO4 and similarly, the product of incomplete decomposition of ammonium-potassium sulfate - NH4KSO4 is KHSO4∙K2SO4.
Conclusion
An increase in the stoichiometric molar ratio in the system «NH4HSO4 ÷ KCl» leads to the formation of more complex hydrated salts and the basic salt of ammonium - ammonium sulfate, and, on the contrary, a decrease in the ratio - to a more basic compound of potassium sulfate.
1. INTRODUCTION
Ammonium sulfate is widely used in various spheres, mainly in agriculture as a fertilizer, as it is a source of nitrogen and sulfur. It contains up to 21% nitrogen, which has a beneficial effect on plants, primarily on the root system of plants [1-3].
Compared to other nitrogen fertilizers such as urea and ammonium nitrate, ammonium sulfate has some agronomic and environmental advantages [4-6]. Researchers [7, 8] have developed a method for obtaining ammonium sulfate in the form of granules, and it is used both individually and as part of compound fertilizers. Obtaining ammonium sulfate by this method decreases the size of granules and increases their strength.
However, despite the known recommendations on the use of ammonium sulfate for growing crops (from potatoes to citruses) on chernozem and sulfur soils, it is used only to a limited extent. The reason for this is the availability of complex fertilizers richer in nitrogen and other useful components. Another reason is the acidic property of fertilizers, which can lead to rapid acidification of the soil. However, the lands of the Mangistau region contain limestone - an alkaline reagent and therefore, the use of fertilizer based on ammonium sulfate will not have a harmful effect on the soil [9].
Unfortunately, we did not find any work on the study of the solid-phase interaction of ammonium sulfate with potassium chloride during the search.All studies on the production of potassium sulfate from various mixtures, such as sodium sulfate and potassium chloride, are devoted to aqueous solutions [10-12]. As an analogue, the interaction between ammonium bisulfate and ammonium chloride can be cited. The reaction products are hydrogen chloride and ammonium sulfate at a temperature below the initial pyrolysis point of 260 °C of the latter compound. The results of the study do not take into account the physicochemical transformations in reaction mixtures before the formation of the final thermally stable compound, potassium sulfate.
Furthermore, it is relevant to obtain double salts on the basis of ammonium sulfate, which is easily assimilated by plants [13].
Ammonium salt-based product range increase is a priority task for the industrial enterprise JSC «KazAzot» (Kazakhstan, Aktau). In order to solve this task, the technology of granulated ammonium sulfate production is being mastered by the enterprise. Despite the fact that the methods of obtaining granules of these fertilizers are known [14-16], the management of the enterprise sets new tasks for researchers – to search for the possibility of using binders based on their own products.
The other objective is to expand the areas of application of products with derivatives based on ammonium sulfate [17-19]. These include combined fertilizers based on ammonium hydrosulfate. The authors have previously studied and given the results of physicochemical analysis of a number of topochemical reactions of the interaction of metal compounds with ammonium sulfate [20].
An analysis of the scientific and patent literature [21, 22] represented that to obtain complex mineral fertilizers as a nitrogen-containing component mainly use ammonium nitrate and urea, which have a high cost. There is limited information in scientific and patent literature on the use of ammonium sulfate for the production of complex fertilizers.
The research aims to provide a scientific basis for the possibility of achieving the set objectives by studying the physicochemical transformations in reaction mixtures for example, to obtain ammonium-potassium sulfate – a fertilizer with three target components.
2. MATERIALS AND METHODS
The experimental research was carried out in a laboratory shaft electric furnace to study the interaction of potassium chloride with ammonium hydrosulfate with observations of changes in the reaction mass and determination of the pH of the gas phase. It was a DTA installation with a transformer and a voltage regulator at a speed of 10 °/minute. The samples were placed in an oven in a crucible made of heat-resistant glass. Additionally, when the maximum temperature is set, the heating is turned off using a thermostat equipped with a chromel-alumel thermocouple.
The samples were placed in the furnace in a crucible made of heat-resistant glass. The required temperature in the furnace was kept constant using a device equipped with a chromel-alumel thermocouple. In all experiments, the sample weight of the substances and reaction mixtures used for the study was calculated according to the assumed reaction equations. Depending on the purpose of the experiment, the sample holding time in the oven was within 60 minutes. The ammonium hydrosulfate of the “clean” label was used for the experiment.
The experimental products were subjected to an X-ray phase analysis on a Miflex 600 diffractometer (Japan) with CuKɑ at a scanning range of 3 °C to 90 °C, and a step of 0.02 Physicochemical changes of the reaction mixtures and some synthesized salts upon heating were studied using a Q-1000/D derivatograph system Q-1000/D by “MOM” F. Paulik, J. Paulik, L. Erdey and LabSys Evo TG-DTA/DTG (Setaram, France), NETZSCH STA 443 F3 in nitrogen atmosphere at a feed rate of 50ml/minute at a heating rate of 10° per minute. The thermodynamic evaluation of reaction probability was obtained using the HCS-5.1 Chemistry (Outokumpu) “Astra-4” software package HCS-5.1 Chemistry (Outokumpu) [23] (Table 1). The appropriate thermodynamic functions of hydrosulfates for which no thermodynamic data was available were calculated by the ionic increment method [24] in order to estimate the probability of their formation.
The comparison of reference data of a number of known compounds, such as ammonium sulfate, with the calculated values of thermodynamic values by the method of ionic increments showed no more than a 1% discrepancy (Table 2).
The charge was made according to the reaction equation with an excess of ammonium hydrosulfate 18.9738g (10.0%), a total of 134.084g, and the mass of potassium chloride in the charge was equal to 74.615g, grade “clean for analysis” with the content of the main substance 99.98%. The total calculated mass of the charge according to the equation of reaction with excess ammonium hydrosulfate was 208.699g. The samples for research were taken from the following mixture.
| Compounds | ∆H, kCal | ∆G, kCal | ∆S, kCal |
|---|---|---|---|
| (NH4)2SO4(cr.) | -1180,0 | -901,3 | 220 |
| NH4HSO4 | -1020,2 | -834,7 | 242,4 |
| (NH4)3H(SO4)2 | - 2215,8 | - 1726,5 | 361,4 |
| NН4K(HSO4)2 | - 2159,2 | - 1871,2 | 472,9 |
| NН4K2(HSO4)3 | - 3298,2 | - 2907,7 | 703,4 |
| NН4K2HS2O8 | - 2431,9 | - 2141,3 | 465,6 |
| (NН4)2KHS2O8 | - 2313,3 | - 1939,5 | 477,5 |
| KНSO4 | - 1139,0 | - 1036,5 | 230,5 |
| NH4KSO4 | - 1292,9 | - 1104,8 | 235,1 |
| K3H(SO4)2 | - 2572,2 | - 2331,9 | 343,9 |
| K3NH4(SO4)2 | - 2704,6 | - 2411,4 | 458,3 |
| K2CO3(cr.) | -1146,1 | -1059,8 | 156,3 |
| K2SO4(cr.) | -1433,7 | -1316,4 | 175,7 |
| K2S2O7(cr.) | - 2069,3 | -1904,4 | 340,5 |
| No. | An Equation of a Chemical Reaction | Трв,°С | ∆Н298 | ∆G298 | ∆S298 |
|---|---|---|---|---|---|
| 1 | NН4HSO4 + KСl = НCl + NН4KSO4 | 205,5 | 71,4 | 43,1 | 96,9 |
| 2 | NH4KSO4 = ½ (К2SO4 + (NH4)2SO4) | 101,5 | - 13,95 | - 4,05 | - 37,25 |
| 3 | ½ (NH4)2SO4 = ½ (NH4HSO4(т) + NH3) | 255 | 56,8 | 24,95 | 107,5 |
| 4 | ½ (К2SO4 + NH4HSO4) = ½ (К2S2O7 + H2О + NH3) | 27,5 | 178,95 | 109 | 118,8 |
| ∑1-4 | NH4HSO4 + KCl = HCl + ½ (К2S2O7 + H2О) + NH3 | 481 | 293,2 | 176 | 388,75 |
| Possible reactions of formation of acid salts | |||||
| 5 | K2SO4 + 2NН4HSO4 = 2KHSO4 + (NН4)2SO4 | - 3 | - 11,2 | 0,7 | - 41,5 |
| 6 | 3NH4KSO4+NH4HSO4 = (NH4)3HS2O8+K3 (HS2O8) + NH3 | -78 | 64,7 | 74 | 332,2 |
| 7 | 3K2SO4 + 2NН4HSO4 = 2K3(HS2O8) + (NН4)2SO4 | - 50 | - 64,8 | 21,1 | -290,1 |
| 8 | (NH4)2SO4 + NH4НSO4 = (NH4)3HS2O8 | - 118 | - 15,6 | 9,5 | - 101 |
| 9 | 3NН4HSO4+NН4K2HS2O8= 2NН4K(HSO4)2 + (NH4)2SO4 | -54,5 | - 5,9 | 1,7 | - 27 |
3. RESULTS AND DISCUSSION
The article presents the research data aimed at creating a technological basis for the processing of metallurgical raw materials using ammonium hydro- sulfate. However, it is possible that these reactions are summarized for successive physicochemical transfor- mations with intermediate products stable up to a certain temperature. Such compounds can include ammonium-metal sulfates - anhydrous salts of Tutton, and hydrosulfates. The calculated values of thermodynamic functions of some complex ammonium salts at 298K are presented in Table 1.
The results of the values of thermodynamic functions of the assumed variants of chemical interaction of potassium salts with ammonium hydrosulfate melt are presented in Table 2.
Since weight loss was calculated as a percentage of potassium chloride, the calculation was carried out according to the reaction equation 1 with respect to the charge, weighing 189.725g. The excess of ammonium hydrosulfate at the temperatures of its decomposition was taken into account separately. The comparative values of calculation by the equations of assumed reactions and data on TG are given in Table 3 and Fig.(1).
| No. | Reaction Equations | t1 – t2, °С | ∆mcalculation % | ∆m fact, % |
|---|---|---|---|---|
| 0 | NH4HSO4(s) = NH4HSO4(l) | 30 – 157.3 | 0 | 0 |
| 1 | NН4HSO4 + KСl = НCl + NН4KSO4 | 157.3–381 | 19.254 | 20.26–1.006 = 19.254 |
| 2 | NH4KSO4 = ½ (К2SO4 + (NH4)2SO4) | 381– 381 | ||
| 3 | ½ (NH4)2SO4 = ½ (NH4HSO4(т) + NH3) [8] | 260 – 360 | 4.4838 | 4.4838 |
| 4 | ½ (К2SO4 +NH4HSO4)=½(К2S2O7+H2О+NH3) | 381 – 450 | 9.2314 | 32.5–23.7378 = 8.7622 |
| ∑ | NН4HSO4+KСl=½ (К2S2O7+H2О)+NH3+НCl | 30 – 450 | 32.9692 | 32.5 |
| 9 | 3NН4HSO4+NН4K2HS2O8=2NН4K(HSO4)2 + (NH4)2SO4 | 260 –190 | 8.0 | 8.95 |
| NН4HSO4 = SO3+NН3+H2O [8] | 450 - 380 | 9.38 | 41.88 – 32.5 = 9.38 |
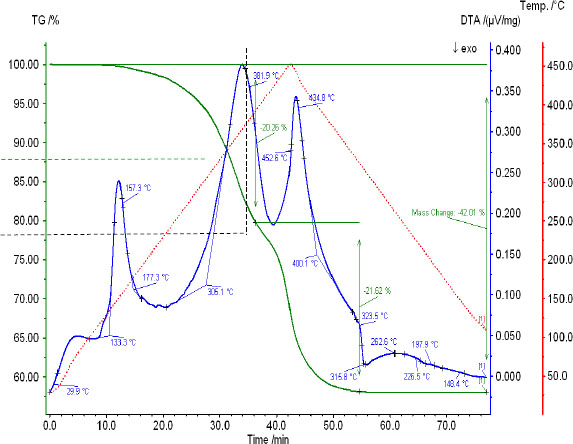
DTA and TG curves of interaction between potassium chloride and ammonium hydrosulfate.
In the temperature range of 125 – 157 °C, the DTA curve has a rectilinear section, characteristic of the melting of ammonium hydrosulfate. In this section, according to TG readings, there is no gas emission.
In the interval of temperatures 157 – 381 °C on the DTA curve, a strong endothermic effect is fixed, which corresponds to the loss of mass by TG 20.26% of the mass of the charge suspension. Furthermore, the calculation according to the equation of reaction 1, the condensed product of the reaction is a double salt - ammonium-potassium sulfate - NH4KSO4, and the mass of released hydrogen chloride by potassium chloride in the charge should correspond to 19.254%. When it decomposes by reaction 2, there should be no loss of mass. Therefore, the difference of 1.006% can be attributed to the beginning of ammonia release as a result of the primary pyrolysis of ammonium sulfate by reaction 3. It should be noted that, in fact, reaction 1 is the initial and limiting reaction for successive reactions 2 and 3, despite the greater probability of the latter occurring. Therefore, as NH4KSO4 appears, its decomposition into constituent sulfates occurs, and in the temperature range of 235 -340 0C, the pyrolysis of ammonium sulfate to the formation of ammonium hydrosulfate and ammonia is completed [25, 26]. It is likely that in the studied mixture, pyrolysis starts earlier due to the release of heat energy via reaction 2.
The amount of gases released as the maximum heating temperature of – 450 °С reached according to the TG curve was 32.5 – 20.26 + 1.006 = 13.246%. Reaction 4 failed to complete at 5.083%.
The additional outgassing occurs at the cooling stage due to the completion of reaction 4 and depends on the stoichiometric ratios of the components of the direct reaction products according to one of the reaction equations 5 - 9. At incomplete pyrolysis of ammonium sulfate, XRA of the cooled residue shows the presence of (NH4)3HS2O8, so the salt formed from 2 ammonium sulfates: (NH4)2SO4∙NH4HSO4 [12]. Depending on the sensitivity of the device and the weight of the sample, the beginning of pyrolysis on the DTA curves is fixed in the range of 230-260 ° C.
The precipitation of such salts from solution is generally known. Similarly, the incomplete decomposition product of ammonium-potassium sulfate - NH4KSO4 is KHSO4∙K2SO4. These salts consist of a basic salt and an acidic salt. Therefore, their formulas should be written as compounds containing an anion such as hydrosulfate and sulfate: (NH4)3HS2O8 and K3(HS2O8). As shown by the DTA data (Table 2 and Fig. 1) of the cooled residues, they contain a compound, double hydrosulfate-2NH4K(HSO4)2, which was formed by reaction 7.
An excess of potassium pyrosulfate of reaction 5 is also retained during cooling. Therefore, because of the lack of ammonium hydrosulfate, the reaction starts with the formation of normal double salt - ammonium potassium sulfate, which, in the course of temperature increase, undergoes successive physicochemical transformations. In this case, at certain temperature intervals, the products can be stable compounds, as well as the probable short-term appearance of thermally unstable acidic salts. The formation of acid salts at the heating stage of potassium salts and ammonium hydrosulfate is the result of the decomposition of double sulfate and their intermediate products, which are accompanied by the formation of ammonium sulfate or hydrosulfates on its basis. Above the pyrolysis temperature of ammonium sulfate, the reactions proceed with the liberation of ammonia and the decomposition of the double acid salts into ammonium hydrosulfate and potassium sulfate. When such a condensed mixture of direct reactions is cooled below 260 0C, acidic salts and ammonium sulfate or double acidic salts are formed again, depending on the stoichiometry of the initial mixtures. The process can continue with the release of ammonia until this temperature is reached.
When the stoichiometric molar ratio of NН4HSO4 ÷ КCl = 2 ÷ 1 increases, the general appearance of DTA curves and mass loss by TG changes (Fig. 2). The reaction mass is 304.82 g, and the calculated amount of released HCl is 36,5 g (11.974% of the charge mass).
The main phases of the cooled residue according to XRA are presented in Table 4.
The ammonium hydrosulfate increase in the charge leads to the formation of salts with more acidic properties, such as ammonium potassium hydrosulfate, in different stoichiometric ratios, depending on the excess of ammonium hydrosulfate and temperature.
According to the TG data (Fig. 2), at 350 °C, the formation of hydrogen chloride was only 73% of the calculated ones (Table 5).
Reaction 1 did not proceed to the end, considering the cooling stage, and the mass loss amounted to 9.93%. An additional amount of ammonia of 1.18% was released before the temperature reached 260 0C, since below that ammonia forms ammonium sulfate with ammonium hydrosulfate. A sufficient excess of ammonium hydrosulfate remains to form ammonium hydrosulfate and ammonium sulfate.
| Phase Name | Formula | Figure of Merit | Phase Reg. Detail | DB Card Number |
|---|---|---|---|---|
| Dipotassium disulfate(VI) | K2(S2O7) | 1.335 | ICDD (PDF-2 Release 2016 RDB) | 01-077-4725 |
| Potassium Ammonium Hydrogen Sulfate | K(NH4)H2(SO4)2 | 1.723 | ICDD (PDF-2 Release 2016 RDB) | 00-020-0850 |
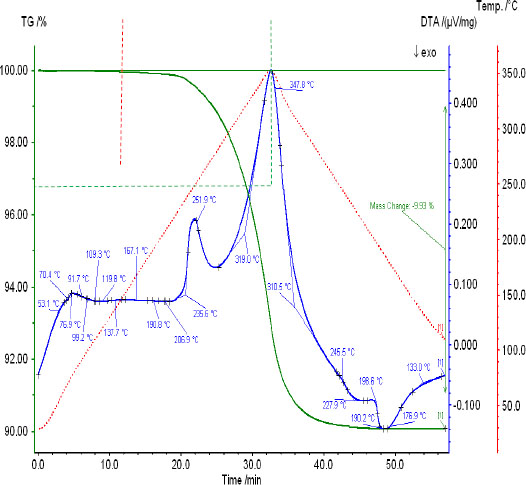
DTA and TG curves of reaction mixture 1 - (NН4HSO4 + KСl), at the molar ratio NН4HSO4 ÷ КCl = 2 ÷ 1.
| No. | Reaction Equations | t1 – t2, °С | ∆mcalculation % | ∆mфакт., % |
|---|---|---|---|---|
| 0 | NH4HSO4(s) = NH4HSO4(l) | 30 – 157,3 | 0 | 0 |
| 1 | NH4HSO4 + КCl = KNH4SO4 + HCl | 157 - 350 | 11.974 | 8.75 |
| Cooling stage (XRA): | ||||
| 15 | NH4HSO4 + KNH4SO4 = NH4K(НSO4)2 + NH3 | 350 – 260 0С | 1,18 | |
| 16 | NH4HSO4 + NH3 = (NH4)2SO4 | ≤ 260 0С | - | |
| Total: | 11.974 | 9.93 | ||
Acid salts are likely to form in the presence of potassium sulfate in the reaction mixtures. The compounds of acid salts can be formed below the temperature of their thermal decomposition, both at the heating of reaction mixtures and at the cooling of condensed products of the direct reaction - metal sulfates and ammonium hydrosulfate (Table 3).
The reaction pyrolysis of ammonium sulfate is well-studied. The physicochemical methods show that the initial temperature point of the process is in the range of 235-2600C [20, 26, 27], depending on the amount of suspension and heating rate at DTA. The mass loss values calculated from the reactions and TG curves comply efficiently (Table 3).
CONCLUSION
The following conclusions can be drawn from the results of the studies:
Thermodynamic functions of complex ammonium salts at 298K have been calculated using reference values of ionic increments.
The values of thermodynamic functions of supposed variants of chemical interaction of potassium salts with ammonium hydrosulfate melt have been calculated.
An analysis of the reaction of the interaction of potassium chloride with ammonium hydrosulfate was carried out, and it was found that:
- In the temperature range of 157 – 260 °C with equal molar ratios NH4HSO4÷KCl, the condensed reaction product is a double salt – ammonium-potassium sulfate (NH4KSO4), and its intensive decomposition into constituent sulfates occurs above 260 °C with simul- taneous pyrolysis of ammonium sulfate to complete formation of ammonium hydrosulfate and ammonia.
- The presence of ammonium hydrosulfate in the direct reaction products leads to the formation of potassium pyrosulfate. At the stage of cooling of condensed products of direct reaction in non-equilibrium conditions, depending on the stoichiometric ratios of components, the formation of hydrosulfates of different compositions is probable.
- In the incomplete pyrolysis of ammonium sulfate, the cooled residue contains (NH4)3HS2O8, i.e., the salt was formed from 2 ammonium sulfates: (NH4)2SO4∙NH4HSO4 and similarly, the product of incomplete decomposition of ammonium-potassium sulfate – NH4KSO4 is KHSO4∙K2SO4.
- The formation of hydrosulfates occurs in the presence of ammonium hydrosulfate in the composition of reaction mixtures, and the greater its proportion, the more acidic the double salts, the formation of which is accompanied by the simultaneous appearance of the main salt - ammonium sulfate;
- At a deficiency of ammonium hydrosulfate, on the contrary, the products of interaction are more basic salts.
- Increase of stoichiometric molar ratio of NH4HSO4 ÷ KCl leads to the formation of more complex hydrated salts and ammonium sulfate.
Thus, the goal of obtaining ammonium-potassium sulfate fertilizer with three target components can be achieved in the form of ammonium potassium sulfate by holding the reaction mixture NH4HSO4 + KCl below its decomposition temperature, preferably in the temperature range 150-200 ° C.
AUTHOR’S CONTRIBUTIONS
All the authors contributed to the development of the concept and design of the study.The material’s preparation, data collection and analysis were performed by Kalkaman Zhumashev, Akmaral Serikbayeva, Akkenzhe Busurmanova, Anar Akkenzheeva, Asia Boranbayeva, Zhansaule Altybayeva, Aigul Gusmanova, Aybala Narembekova, Feruza Berdikulova, Arman Mauleshev, and Tugelbai Kenbayev. The first version of the manuscript was written by Kalkaman Zhumashev and Akmaral Serikbayeva, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that they have no conflict of interest.
LIST OF ABBREVIATIONS
| TG | = Thermogravimetry |
| DTA | = Differential thermal analysis |
| DTG | = Differential thermal gravimetry |
| cr. | = Crystal |
| ∆H kCal | = Enthalpy |
| ∆G | = kCal - Gibbs energy |
| ∆S kCal | = Entropy |
| Трв | = Temperature equilibrium |
| XRA | = X-ray phase analysis |
| t1-t2, °С | = Temperature |
| ∆mcalculation | = Calculated weight difference |
| ∆m fact | = Actual weight difference |
| s | = Solid |
| l | = Liquid |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.


