All published articles of this journal are available on ScienceDirect.
Investigation of the Process of Agglomeration of Phosphorites Using Phosphate-Siliceous Shales and Oil Sludge
Abstract
Introduction
This article aims to discuss the physico-chemical features of the agglomeration process of phosphorus fines using phosphate-siliceous shales and oil sludge.
Methods
The composition and structure of the starting materials and physico-chemical transformations under thermal influence are studied using IR spectrometry and differential thermal analysis methods.
Results
The results of IR spectrometric analysis of the phosphate siliceous shales are characterized by intense peaks at 493.78, 547.78, and 678.94 cm-1, corresponding to Ca-O-P compounds. Moreover, the wave oscillations in the region of 837.11-995.27 cm-1 indicate the characteristics of Si-O valence bonds, and in the region of 1114.86-1431 cm-1 depict the characteristics of Si-O-Al compounds. The IR spectrum of oil sludge is characterized by the presence of wave oscillations in the region of 1411.89-2904.80 cm-1 corresponding to petroleum components.
Conclusion
The differential thermal analysis of the investigated sample of phosphate-siliceous shale does not have intense endo- and exo-effects, and it is characterized by a significant predominance of hydrate compounds of aluminosilicate and carbonate components.
1. INTRODUCTION
The intensive development of the phosphorus industry in Kazakhstan has led to the gradual depletion of rich phosphate raw materials in terms of the main component (P2O5), and the oil refining industry has led to the accumulation of hydrocarbon waste of complex minera- logical and chemical compositions in dumps. The use of raw phosphate materials in the electrothermal production of phosphorus requires the improvement of preliminary thermal preparation, including agglomeration firing. Our proposed improvement of agglomeration roasting of phosphorus raw materials using phosphate-siliceous shales and human-made waste, oil sludge, and petroleum coke will help to reduce the cost of raw materials and fuel, which will increase the technical and economic indicators of electro- thermal phosphorus production.
Kazakhstan has been the only state in the Commonwealth of Independent States with a developed phosphorus production industry in recent years. This is due to the location of the richest deposits of phosphorus ores in the foothills of Karatau. The deposit is characterized by a complex genesis with transformed dolomites and phosphates of the Chulaktau formation, overlapping rocks of the Malokaroy, Zhaanatas series, and Shabaktin formation, carbonated shales, sandstones, as well as secondary calcite [1-6].
The Karatau Phosphorite-Bearing Basin (KPBB) is located in the territory of Zhambyl and Turkistan regions, and it is a 120 km long strip stretching in a north-westerly direction. More than 40 deposits of phosphorite ores with total estimated reserves of about 15 billion tons of ore have been identified within the KPBB. Analysis of the state of phosphorite reserves showed that substandard ores of carbonate, siliceous-carbonate, and carbonate-siliceous composition predominate in most formations [7-14].
Carbonate phosphorites, phosphate-siliceous shales, a significant part of siliceous-carbonate, and siliceous ores in composition do not meet the requirements of the mineral fertilizers industry (P2O5>24.5%) and require preliminary preparation.
High-quality ores account for about 10% of the total reserves of different types of phosphate raw materials in the Karatau basin. The ore consists of microscopic grains of phosphate matter and oolites cemented with phosphate or carbonate in most cases. Phosphorites are difficult to pre-concentrate in an aqueous medium due to finely crystalline, interspersed phosphate particles enriched without obtaining dispersion [15-27].
Phosphorite is the main raw material used in the electrothermal production of phosphorus and its compounds. Therefore, it is necessary to carry out the agglomeration process after the enrichment of the phosphorite concentrate, taking into account the decrease in reserves of rich phosphorite lump ore [6, 28, 29].
In the agglomeration roasting, phosphorus fines are dipped in a grate at the Novodzhambul Phosphorus Plant (NDPP), Kazphosphate LLP. It is the only enterprise that produces yellow phosphorus. The problem of using a huge amount of phosphorous fines for electrothermal processing has been solved with the development of agglomeration technology [30].
Phosphorites consist of 4 main phases: fluorapatite, hydroxyapatite, quartz, and dolomite [31]. It has been observed that in the agglomeration firing of phosphorites, the yield of durable agglomerate increased with an increase in the carbon dosage to 7%. The formation of Ca2MgSi2O7 increased with increasing temperature and silica content. The agglomeration mechanism during the sintering of phosphorite should include the processes of “sintering” and “pelletising”.
The analysis of the thermophysical characteristics of the agglomerate of phosphorous ore material was carried out, taking into account the peculiarities of the operation of agglomeration machines in the research by a group of scientists, Bobkov V.I., Orekhova V.A., and others [32]. It was found that the regular regime is a suitable method for determining the coefficients of thermal conductivity and thermal conductivity in agglomerates of phosphorus-containing ore raw materials. This fact is important in the study of natural ore rocks characterized by significant heterogeneity of structure and composition [26, 33, 34].
Technogenic mineral formations of flint dumps have been formed during the development of the Zhanatas, Kokjon, Kistas, and Tiesaiphosphorite deposits. The dumps are located in the territory of the Sarysu and Talas districts of the Zhambyl region and the Suzak district of the Turkistan region. Mining operations from the dump of flints, phosphate-siliceous shales, and substandard ores, their primary processing, crushing, and sorting have been carried out in the branches of Kazphosphate LLP, Karatau Mining Complex, and Chulaktau Mining and Processing Complex, using existing infrastructure and equipment [35-38].
The observed tendency of deterioration of the quality of phosphate raw materials in the absence of improved preparation methods leads to a decrease in the technical and economic indicators of electrothermal phosphorus production and the formation of a significant amount of waste [39-43].
Furthermore, the choice of flux is based on the phenomenon that when an oxide with opposite chemical properties is added to another oxide, the melting point of the system significantly decreases [44-51].
The target process in the phosphorus furnace is the reduction of phosphorus from phosphate raw materials. This process is successful only in the presence of silica, which binds CaO released from phosphates into silicates. Therefore, it is necessary to introduce silica as a flux. The modulus of acidity (MA), equal to the ratio of the sum of acidic (SiO2 + Al2O3) to the sum of basic (CaO + MgO) oxides, also determines the physicochemical properties of the final slags.
The economic prerequisites for the use of phosphatized flints are that, along with silica, additional resources of P2O5 are included in the production of phosphorus.
In addition to ores and phosphatized flints, the Karatau deposit contains large reserves of poor (off-balance) phosphorite ores (with a P2O5 content of 19-21%), represented by siliceous and pelitomorphic siliceous phosphorites. The latter can also be considered fluxing additives in the electrothermal processing of conditioned phosphorites [6].
A significant amount of oil sludge is formed, which is a mixture of various mechanical impurities (both mineral and organic origin), petroleum products, and water as a result of the production activities of oil refineries.
An analysis of the productive activities of a refinery reported that 8-12% of mechanical impurities come from oil, 20-25% from fresh water, and 65-70% are formed at the refinery itself. Oil sludge from sewage treatment plants is collected in sludge accumulators, which also receive oil emulsion from the trap oil system, stripping tanks, and containers [52].
In earlier studies, several technologies were proposed for the agglomeration of phosphate fines using other hydrocarbon-containing wastes as a partial substitute for coke [53-57]. An article envisaged the use of poor phosphoritic and phosphate-siliceous oil shale, as well as oil sludge, as a substitute for sintering fuel.
Particular attention has been paid to the agglo- meration of phosphorites by using a method of calculating the limiting and optimal conditions of the chemical-energy process. There are known works on the agglomeration of phosphorites using boron-containing compounds as a fluxing component that helps to reduce the sintering temperature [58].
The introduction of oil sludge into the charge to partially replace the fuel component of coke will help reduce the cost of the agglomerate and solve environmental problems in the region [55, 57, 59-66].
The purpose of our research is to optimize the agglomeration process of non-standard phosphorites using phosphate-siliceous shale and oil sludge as a fluxing component and a substitute for coke. The proposed improvement contributes to the disposal of substandard dumps of non-standard raw materials and waste.
2. MATERIALS AND METHODS
The samples of non-standard phosphorites and phosphate-siliceous shales from the Zhanatas field and Petro Kazakhstan Oil Products and oil sludge were selected as materials for research.
Samples of non-standard phosphorite and phosphate-siliceous shales from the Zhanatas deposits for use in agglomeration firing were pre-milled in a laboratory ball mill to a piece diameter of less than d≤0.1 mm. Then, the ground samples were dried in a drying cabinet at a temperature of 100°C.
The oil sludge sample had a complex consistency of an oiled mineral phase with a humidity of about 20%. Therefore, it was dried at a temperature of 100-120°C for 60 minutes before use.
The special modern devices of the regional engineering laboratory “Structural and Biochemical Materials” were used to study the physicochemical characteristics of the initial raw materials, charge compositions, and final products.
The microstructure of the initial samples under study was examined using a JEOL JSM 6490 LV scanning low vacuum electron microscope (Japan) with INCA Epegdu 350 energy dispersive microanalysis systems (Oxford Instruments, UK) and HKL Basic polycrystalline sample texture analysis system (Oxford Instruments, UK). The morphology of the samples was studied using a JEOL JSM – 6490 LV scanning low-vacuum electron microscope with INCAEnergy 350 energy dispersive microanalysis systems (Oxford Instruments) and HKL Basic polycrystalline sample texture analysis system (Oxford Instruments).
The study of the content of organic compounds in oil sludge was carried out by thin-layer chromatography using SPECORD IR-75.
The Shimadzu IR Prestige-21 Infrared Fourier spectrometer was also used with the plate for the disturbed total internal reflection (Miracle by PIKE Technologies).
Thermal analysis of the initial samples of phosphate-siliceous shales and oil sludge was carried out on a derivatograph of F. Paulik, D. Paulik, and L. Erdei systems (“MOM”, Hungary). The shooting was carried out in an air environment, in the temperature range of 20-1000°C and in a dynamic heating mode at dT/dt = 10. Calcined Al2O3 weighing 500 mg was used as a reference substance, with a scale sensitivity of 500 µV to measure the weight change of the sample. The sensitivity of other measuring systems of the device was as follows: DTA=250 µV, DTG=500 µV, TG=500 µV, and T =500 µV.
The thermodynamic parameters of possible reactions in the temperature range of 298-2000 K, using the “HSC Chemistry” program, were employed to study the process of decarbonization of phosphorous raw materials using oil sludge.
The thermodynamic probability of the reaction was determined by the change in Gibbs free energy (ΔG) using the following equation:
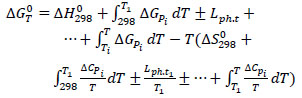 |
(1) |
The mathematical planning of the experiment was performed on the basis of thermodynamic data obtained from the MatLab program using a second-order equation, taking into account the Student’s t-test and Fisher’s criteria.
3. RESULTS AND DISCUSSION
3.1. The Results of the Analysis of the Initial Samples
The results of the chemical analysis of the initial phosphate-siliceous shales sample of the Zhanatas phosphorite deposit showed that the content of P2O5 ranged from 2.4 to 11.2%, SiO2 from 63.4 to 79.8%, and CaO from 3.1 to 17.3%. Moreover, the ratio of P2O5 / CaO was 0.89 (Table 1).
The IR spectrometric analysis of phosphate siliceous shales from the Zhanatas deposit is shown in Fig. (1). Available information and reference data of inorganic and organic compounds were used to identify the results of IR spectrometric analysis [67, 68].
| Indicators of MTC | Contents of MTC | Actual Values according to the Test Data | ||
|---|---|---|---|---|
| Max | Average | Min | ||
| SiO2 | at least 70% | 63.4 | 79.8 | 92.8 |
| 3.8 P2O5+1.1 | at least 100% | 91.9 | 109.7 | 117.0 |
| СаО | not standardized | 3.1 | 9.5 | 17.3 |
| Fe2O3 | not standardized | 1.0 | 1.0 | 2.1 |
| P2O5 | no more than ± 1.5 | 11.2 | ±6.6 | 2.4 |
| Granulometry | no more than 100-150 mm | - | 14.8 | 30.0 |
| A trifle 0-10mm | no more than 10% | 2.6 | 14.8 | 30.0 |
IR spectrum of the phosphate-siliceous shales from the zhanatas deposit.
The analysis of the obtained absorption spectrum of the phosphate siliceous shales sample was characterized by intense peaks at 493.78, 547.78, and 678.94 cm-1, corresponding to Ca-O-P compounds. The absorption spectrum in the range of 837.11-995.27 cm-1 indicated characteristics of Si-O bond fluctuations. Furthermore, wave oscillations in the region of 1114.86-1431 cm-1 corresponded to Si-O-Al valence bonds, and non-intense peaks in the region of 1616.35-2360.87 cm-1 indicated characteristics of O-H bonds.
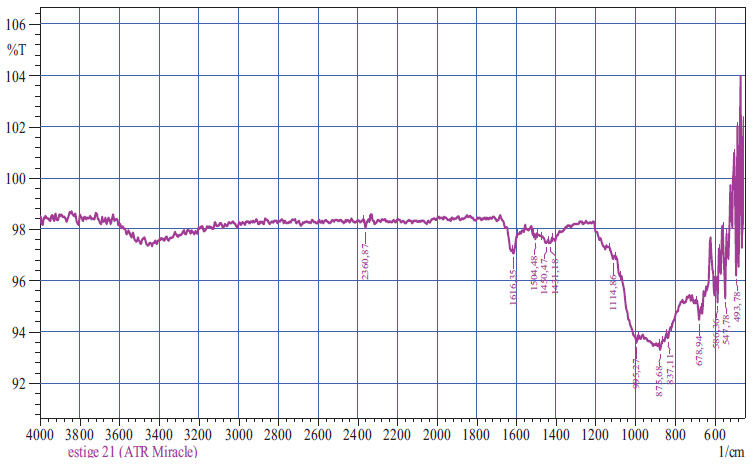
The results of the IR spectrometric analysis of oil sludge are shown in Fig. (2).
The results of IR spectrometric analysis of oil sludge showed intense peaks in the regions of 594.08- 1080.41cm-1, indicating the characteristics of Si-O-Al and Si-O bonds. The spectrum in the region of 1411.89-2904.80cm-1 corresponded to the petroleum products, 1411.89 cm-1 corresponded to the C-C chains of the benzene ring, 1612-1766 cm-1 corresponded to the aliphatic chains of CH3, and 2364.73-2904 cm-1 corresponded to the bonds of C-H alkyl groups.
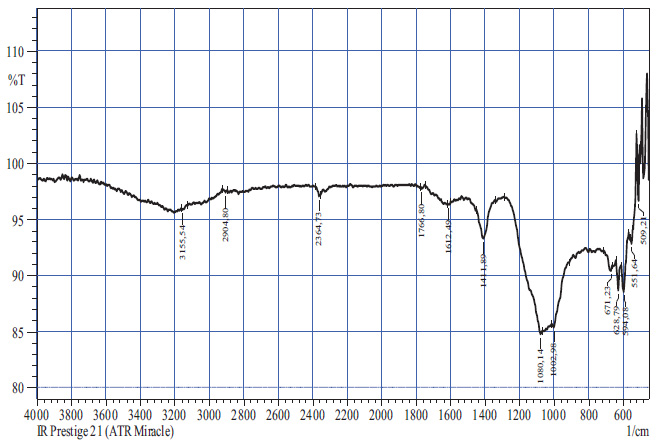
The Differential Thermal Analysis (DTA) of the initial samples of phosphate-siliceous shales and oil sludge are shown in Figs. (3 and 4).
The IR spectrum of the oil sludge.
DTA of phosphate-siliceous shales.
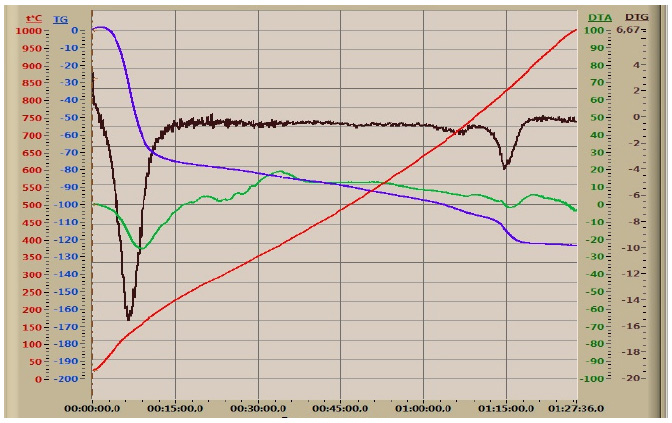
DTA of oil sludge.
The DTA analysis of the phosphate-siliceous shales from the Zhanatas deposit demonstrated a significant loss of sample weight at a temperature of 120°C and the gas formation characterized by an endo-effect at 160-180°C. Significant exo-effects were observed at 230, 420, and 900°C with a further increase in temperature. The first two were associated with the burning out of the organic components of phosphate-siliceous shales and, at 900°C, the decomposition of sulfate-sulfide components.
The endothermic effect in the 160°C region demons- trated the removal of total moisture with the active release of a gaseous component and subsequent weight loss, as indicated by the graphical data from the DTA. A stepwise exothermic effect was observed between 280 and 400°C, indicating the combustion of organic components. At 830°C, an endothermic effect was observed, indicating the decomposition of carbonate components in the mineral portion of the oil sludge [69].
3.2. Decarbonization of Calcium Carbonate during Agglomeration Firing
There is evidence that the decarbonization of CaCO3 was significantly accelerated in contact with SiO2 and Al2O3 [35, 36]. Thus, the temperature of the onset of calcite dissociation in the air decreased to 610°C due to a solid-phase reaction in the presence of Al2O3 and SiO2, as presented in Eqs. (2, 3):
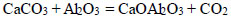 |
(2) |
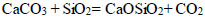 |
(3) |
In this regard, it is of interest to study the thermodynamic probability of the formation of calcium silicates and aluminates in the presence of hydrocarbon groups.
Such groups of hydrocarbon compounds, including CH4, C2H4, C2N6, C4H40, and C6H6, as well as mineral compounds, such as SiO2, Al2O3, MgO, and CaO, were determined in the composition of oil sludge, which was in accordance with the results of physico-chemical and chemical studies [70].
Thermodynamic analysis can provide significant information about the decomposition of carbonate compounds in the presence of oil sludge components.
The calculation of the Gibbs energy change was carried out using the exact entropy method, taking into account the phase and modification changes in the components of the following reactions (Eqs. 4-15):
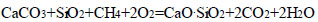 |
(4) |
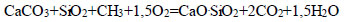 |
(5) |
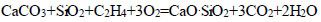 |
(6) |
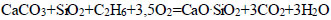 |
(7) |
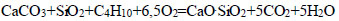 |
(8) |
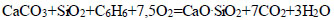 |
(9) |
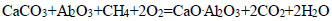 |
(10) |
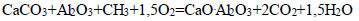 |
(11) |
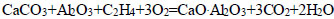 |
(12) |
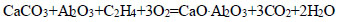 |
(13) |
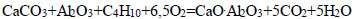 |
(14) |
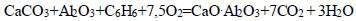 |
(15) |
Based on the performed calculations of the Gibbs energy, the general pattern for the decarbonization of CaCO3 involves the thermodynamic probability of forming calcium silicates and aluminates in the presence of all studied organic compounds within the temperature range of 298-1500 K. This is evidenced by the negative values of the Gibbs energy. The results of the Gibbs energy calculation are shown in Fig. (5).
The thermodynamic probability of decomposition of calcium carbonate with the formation of calcium monosilicate varied in the presence of hydrocarbons, as shown in the following series: CH4<СН3<С2Н4< C2H6<C4H10<C6H6. The maximum negative value (-3350.48 kDJ/mol, at T=1500 K) indicates the decarbonization of CaCO3 with the formation of CaO.SiO2 in the presence of C6H6 (Eq. 8).
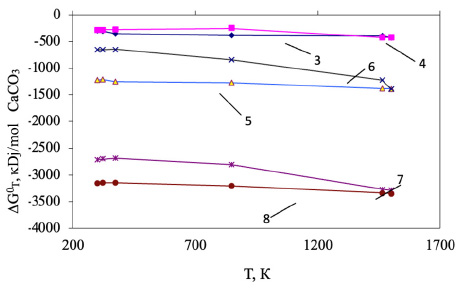
The effect of temperature on CaCO3 – SiO2 – CnHm systems.
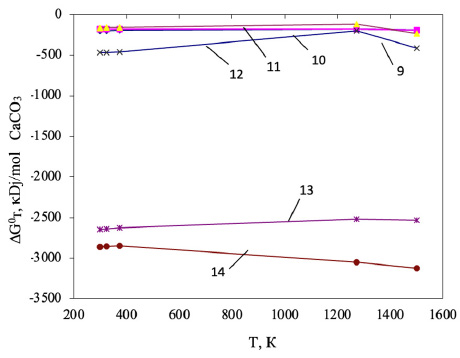
The effect of temperature on CaCO3 – Al2O3 – CnHm systems.
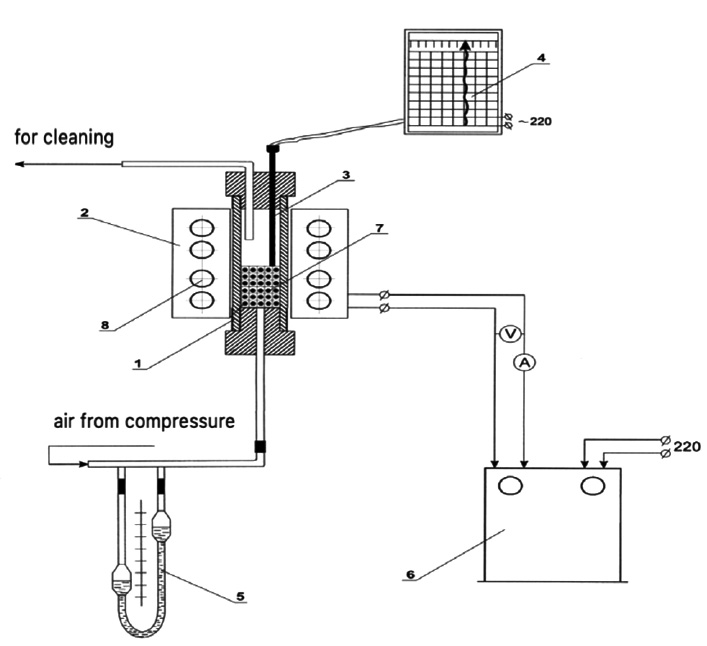
Installation diagram for the study of decarbonization of phosphorites in the presence of hydrocarbons (1 – quartz reactor; 2 – electric furnace; 3 – thermocouple PP-1; 4 – automatic potentiometer KSP-4; 5 – rheometer; 6 – RNO transformer; 7 – basket with granules; 8 – carborundum heaters).
Decarbonization reactions of calcium carbonate with the formation of aluminate in the presence of the above-mentioned carbon compounds are thermodynamically probable, as evidenced by negative Gibbs energy values in the temperature range under study (Fig. 6).
The maximum negative value of ΔGто was observed during the decomposition of CaCO3 to form CaO. Al2O3 in the presence of C6H6 (-3122.52 kJ/mol, at T = 1500 K).
3.3. Mathematical Processing of Thermodynamic Data
The sintering process of charge materials occurs by the movement of gas through the charge layer, and therefore, the gas affects almost all of the parameters. In accordance with the basic laws of the process, the productivity of sintering plants is directly dependent on the amount of gas sucked through the sintered layer per unit of time [71, 72]. Therefore, increasing the air filtration rate through the layer by reducing its gas dynamic resistance is the main way to intensify the sintering process.
The experimental determination of the sintered layer during agglomeration of phosphorite fines and phosphate-siliceous shale from the Zhanatas field in the oil sludge was carried out (Fig. 7). The material was loaded into a metal basket and placed on a grate in the firing zone. The heated air was supplied under the grate, which was filtered through a layer of material and participated in firing. The temperature in the firing zone was controlled using an automatic self-recording potentiometer KSP-4.
The most important factors determining the gas permeability of the charge for the entire course of the agglomeration process are the amount of moisture and fuel in the charge, the optimal values of which correspond to the best indicators of the decarbonization process and the quality of the agglomerate.
The optimal amount of moisture in the charge corresponded to 8%. The coke content in the charge varied from 4.5 to 8.0%. Petroleum sludge was used as an additional fuel to obtain a given acidity modulus. The yield of the 10 mm fraction suitable for further electrothermal processing was 50.5-56.6%.
The starting materials were pre-crushed to a fraction < 0.07 mm and thoroughly mixed until complete homogenization. Then, granules with a diameter of 8-10 mm were prepared on a bowl granulator and were dried in a drying cabinet. The samples were ground in an agate mortar and analyzed for unbound calcium oxide after firing. The degree of decarbonization was determined by the content of CaO in the samples after firing. The content of calcium oxide was calculated using the following formula (Eq. 16):
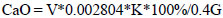 |
(16) |
Where V is the amount of KMnO4, ml; 0.002804 is the coefficient for recalculation; K is the correction to the titer of 0.1 N KMnO4 solution; 0.4 is the coefficient taking into account the determination from the aliquot part of the solution or dilution of the solution, and G is a dry sample in grams.
Currently, mathematical and computer modeling is necessary due to the development of technology, and processes have become increasingly computerized in chemical technology enterprises.
Currently, the foundations of the methodology of scientific research, including mathematical modeling and computational experiments, have already been formed. The essence of this methodology is to replace the original object with its mathematical model and further study it with modern computing tools. The method of mathe- matical modeling using the results of thermodynamic calculations covers new areas, from the development of large technical systems and their management to the analysis of the most complex technological indicators of processes [73-77].
The obtained results of calculating the Gibbs energy and the graphical dependences of the studied reactions indicate the thermodynamic probability of decarbonization reactions in the studied SiO2/CaCO3 ratios. The task was set to determine the shape of the response surface of the interaction under study by using the method of rotatable planning of the second order by using the obtained results of the influence of m and temperature on the change in the Gibbs energy of decarbonization of calcium carbonate. The intervals in the change of m and temperature (T) are presented in Table 2. A change in the Gibbs energy of decarbonization of calcium carbonate was chosen as the target output variable (response surface).
The initial data for planning experiments in encoded and natural form are presented in Table 3.
The plan for conducting studies on the effect of the SiO2/CaCO3 ratio and temperature (T, K) on the Gibbs energy of decarbonization of calcium carbonate is presented in Table 4.
The regression equation was based on the studies carried out according to the plan (Eq. 17):
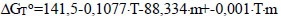 |
(17) |
The deviation of the calculated result from the experimental one does not exceed 4.0%, which indicates good convergence, as presented in Table 4. Checking the significance according to the Student's criterion showed that all the coefficients turned out to be significant. The use of Fisher’s criterion confirmed the adequacy of the mathematical model. The shape of the surface has an inclined plane. The horizontal sections of the surface are shown in Fig. (8).
| Reactions | m | ∆GTo(KDj/mol CaCO3) at Т,К | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 273 | 473 | 573 | 773 | 873 | 1073 | 1173 | 1273 | 1373 | 1473 | 1500 | ||
| CaCO3=CaO+CO2 | 0 | 134.5 | 102.7 | 87.2 | 56.7 | 41.7 | 12.4 | -2.0 | -16.2 | -30.1 | -44.0 | -47.6 |
| CaCO3+SiO2=CaSiO3+CO2 | 1 | 44.7 | 12.5 | -3.3 | -34.1 | -49.1 | -78.5 | -92.8 | -106.9 | -120.8 | -134.7 | -138.4 |
| 2CaCO3+SiO2=2CaO∙SiO2+2CO2 | 0,5 | 66.8 | 34.7 | 19.1 | -11.6 | -26.6 | -56.2 | -71.0 | -86.0 | -100.8 | -115.6 | -119.5 |
| 3CaCO3+SiO2=3CaO∙SiO2+3CO2 | 0,3 | 95.4 | 62.8 | 46.8 | 15.3 | -0.14 | -30.6 | -45.6 | -60.4 | -75.1 | -89.7 | -93.6 |
Table 3.
| Factors | Coded View | Natural View | ||
|---|---|---|---|---|
| Х1 | Х2 | Т | m | |
| Lower level | -1 | -1 | 273 | 0 |
| Upper level | +1 | +1 | 1473 | 1 |
| Zero level | 0 | 0 | 873 | 0.5 |
| The variation interval | Δ | Δ | 600 | 0.5 |
| Shoulder + α | +1.44 | +1.44 | 1500 | 0 |
| Shoulder - α | -1.44 | -1.44 | 0 | 0 |
| Nº samples | Т,К | m | ∆GTоexp | ∆GTоcalcul | Deviation,% |
|---|---|---|---|---|---|
| 1 | 273 | 0 | 112 | 112.0979 | 0.087 |
| 2 | 1473 | 0 | -17.1 | -17.1421 | 0.246 |
| 3 | 273 | 1 | 23.4 | 23.4909 | 0.388 |
| 4 | 1473 | 1 | -106.9 | -106.9491 | 0.046 |
| 5 | 1500 | 0.5 | -106.3 | -106.321 | 0.020 |
| 6 | 1373 | 0.5 | 112.06 | 112.06984 | 0.009 |
| 7 | 873 | -1.341 | 167.1 | 167.10449 | 0.003 |
| 8 | 873 | -0.341 | 77.8 | 77.89748 | 0.125 |
| 9 | 873 | 0,5 | 2.8 | 2.8744 | 2.657 |
| 10 | 873 | 0,5 | 2.77 | 2.8744 | 3.769 |
| 11 | 873 | 0.5 | 2.8 | 2.8744 | 2.657 |
| 12 | 873 | 0.5 | 2.77 | 2.8744 | 3.769 |
| 13 | 873 | 0.5 | 2.8 | 2.8744 | 2.657 |
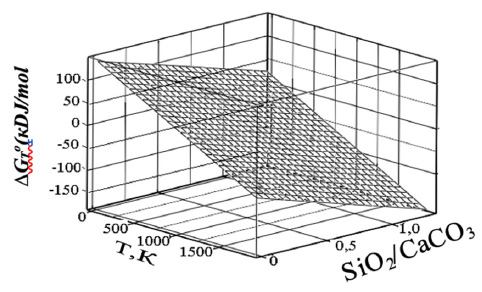
The effect of the SiO2/CaCO3 ratio and temperature on the shape of the surface- ∆GTodecarbonization.
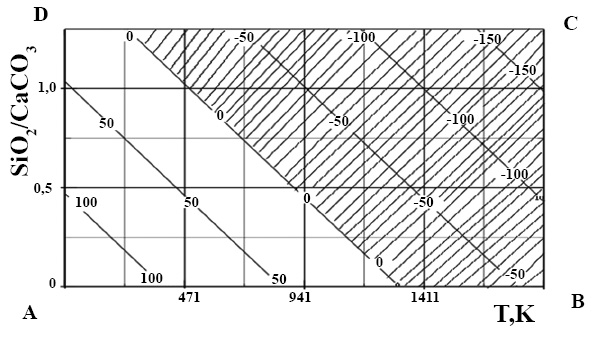
The effect of the SiO2/CaCO3 ratio and temperature on the shape of the isolines - ∆GTodecarbonization.
The simultaneous effect of temperature and m on the decarbonization of calcium carbonate is characterized by a temperature above 800 K with a ratio of m equal to 3, as follows from the volumetric dependence.
The effect of the SiO2/CaCO3 ratio and temperature on ∆GTo is shown in Fig. (9).
The shape of the isolines of the horizontal section of the volumetric graphical data showed the beginning of the decarbonization of calcium carbonate along the D-B-C line and then in the A-B-C-D region. Moreover, an increase in the SiO2 content in the raw material reduced the temperature at the beginning of decarbonization to 1280K.
CONCLUSION
In this study, the characteristics of phosphorite ores and related rocks, as well as their lithology, mineralogical structure, and reserves, were discussed. Furthermore, the characteristics of oil refining waste were summarized based on an analytical review of information data and a patent search. The current state of phosphorus production was studied, and shortcomings and methods of improving agglomeration roasting of phosphorus raw materials using various fluxing components were identified.
The chemical and mineralogical features of phosphate-siliceous shales and oil sludge were studied using physico-chemical analysis methods.
The results of IR spectrometric analysis of the phosphate-siliceous shales from the Zhanatas deposit showed that intense peaks in the region of 493.78, 547.78, and 678.94 cm-1 corresponded to the valence compounds of calcium phosphate Ca-O-P. Absorption spectra in the region of 837.11-995.27 cm-1 demonstrated the character- istics of the Si-O bond, and wave oscillations in the region of 1114.86-1431 cm-1 corresponded to Si-O-Al valence bonds. Moreover, non-intense peaks in the region of 1616.35-2360.87 cm-1 demonstrated the characteristics of O-H bonds. The results of the analysis of oil sludge demonstrated intense absorption waves in the region of 594.08 and 1080.41 cm-1, indicating the valence bonds of Si-O-Al and Si-O compounds. The spectra in the region of 1411.89-2904.80 cm-1 corresponded to the petroleum product, 1411.89 cm-1 corresponded to the C-C chains of the benzene ring, 1612-1766 cm-1 corresponded to the aliphatic chains of CH3, and 2364.73-2904 cm-1 corresponded to the bonds of C-H alkyl groups.
Calculations of the thermodynamic characteristics of possible reactions corresponding to the working composition of the charge, phosphorite, phosphate-siliceous shale, and oil sludge in the temperature range of 298-15000C demonstrated negative Gibbs energy values, indicating the thermodynamic probability of all studied reactions.
The components of organic compounds of oil sludge were determined based on the physico-chemical analysis using thin-layer chromatography. Groups of maltenes, asphaltenes with inclusions of toluene resins, and gas oil compounds were identified by the characteristic absorp- tion spectra of the oil sludge sample.
The results of mathematical planning for decarbonization of the working charge at SiO2/CaCO3 mass ratios from 0.3 to 1 in terms of Gibbs energy corresponded to the response surface -(∆GTo) interaction of the components. The significance test, according to the Student's criterion, demonstrated that all coefficients turned out to be significant and were characterized by minor deviations in experimental and calculated data. The use of Fisher’s criterion confirmed the adequacy of the mathematical model. These data were confirmed by volumetric graphical dependencies along the A-B-C line and then in the A-B-C-D region. Moreover, with an increase in the SiO2 content in the charge, the temperature at the beginning of decarbonization decreased.
The results obtained are of great practical importance for optimizing the technological parameters of the agglomeration process using organic wastes.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| KPBB | = Karatau Phosphorite-Bearing Basin |
| NDPP | = Novodzhambul Phosphorus Plant |
| MTC | = Modern Technical Conditions |
| DTA | = Differential Thermal Analysis |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.


