All published articles of this journal are available on ScienceDirect.
The Development of A Technology for the Purification of Sodium Chloride by Removing Impurities Using the Phosphate Method
Abstract
Background
Sodium chloride is widely used in medicine, food production, and chemical manufacturing, where product purity is critical. While high-purity NaCl can be obtained through existing methods, these often incur significant costs. Therefore, it is important to develop a more practical and cost-effective purification process. This study investigates the purification of table salt through the removal of insoluble residues and impurity ions using phosphate treatment.
Methods
Halite ore from the Bakhyt-tany deposit was processed. The study employed infrared spectroscopy (IR), scanning electron microscopy (SEM), and spectrometric analysis to assess purity levels and impurity removal efficiency.
Results
The optimal liquid-to-solid phase ratio and mixing time were identified. The stoichiometric amount of sodium phosphate required to remove calcium and magnesium ions was established, achieving up to 99% impurity removal.
Discussion
The phosphate method proved effective in significantly reducing bothт soluble and insoluble impurities in sodium chloride. The separation of precipitated impurities by sedimentation and subsequent spray drying of the purified solution provides a viable, cost-efficient alternative to conventional methods. Further investigation is needed to assess the scalability and environmental impact of the process.
Conclusion
The results enabled the development of a technological scheme for purifying sodium chloride using the phosphate method, offering high efficiency and practical applicability.
1. INTRODUCTION
Sodium chloride (NaCl) is obtained by drying salt solutions and extraction from brines. Processing the mineral on the surface of the earth is economically more profitable than mining, as evidenced by the example of Kazakhstan, which is one of the largest producers of NaCl in Central Asia, producing more than 1 million tons annually, of which about 40% is exported. There are more than 2500 salt lakes in the country [1, 2]. However, the quality of salt obtained by the fractional crystallization method is reduced by the presence of impurities (calcium, magnesium, sulfates, bromides, iodides), which necessitate their removal to meet industrial standards [3, 4]. The cost-effectiveness of solar salt production from natural brines is well-documented, but effective methods of impurity purification are essential. High-quality industrial salts can reduce the cost of brine purification and wastewater formation. It is important to note that methods to produce high-purity NaCl are often complex and costly. Recrystallization is an effective method of reducing calcium, magnesium, sulfate ions and trace elements [5, 6]. In addition to soluble impurities, natural salt contains insoluble residue (sludge) that requires removal, and the choice of desliming method depends on the content of insoluble impurities in the ore [7]. In the process of desliming ore, there is a possibility that undesirable impurities may be incompletely removed, thereby resulting in a deterioration of the quality of the product. Slimes have been observed to exert a detrimental effect on the filtration and dewatering processes by absorbing a significant proportion of the reagents that have been introduced [8]. In the context of flotation beneficiation of ores, desliming constitutes a pivotal step involving the removal of insoluble sludge from the process cycle [9, 10]. Hydromechanical desliming has been shown to be effective only for particles with weak binding, whereas ultrasonic treatment (UST) has been demonstrated to enhance the efficiency of insoluble residue removal, as evidenced by studies on phosphorus-bearing and sylvinite ores [11, 12]. However, conventional purification methods such as lime-soda and barium carbonate are associated with several drawbacks, including low efficiency, the toxicity of barium, and the risk of pneumoconiosis [13-15]. The use of concentrated HCl for purification can lead to corrosion of equipment and the necessity of expensive washing of crystals with high-purity water, resulting in increased costs [5, 16-18]. Although NaCl can be purified to a high degree, the selection of a practical and economical method is of significance [19]. The aim of this paper is to study the purification technology of table salt and its environmental impact. Sodium phosphate, a widely employed reagent in water softening and calcium and magnesium ion precipitation, is considered a significant component [18, 20, 21]. The phosphate method offers several advantages: phosphate compounds are widely used in the food industry, and, in small quantities, do not pose a danger to human health. Natural salt mineral of Bakhyt-Tany deposit of Sozak district of South-Kazakhstan region was chosen as an object of research. Natural sodium salt mineral in the selected deposit is characterised by an amount of impurities and location on the ground surface. The Bakhyt-Tany deposit is located 15 kilometres from the Tasty settlement. Lake Bakhyt-Tany is a continental dry lake (it retains surface brine only in the wet season). By composition, it is chloride without root salt. The surface area of the lake is 1.87 km2. The surface is characterised by a flat, smooth topography, with evidence of previous mining activities. In the vicinity of the shoreline, the deposit is observed to be swollen and fractured, with the presence of liquid silt extruding through the fissures, forming accumulations up to 5-8 cm in height on the surface. In general, the salt deposit can be described as a layer that gradually extends outwards from the lake's periphery. The water is characterised by a chemical composition comprising predominantly sulphate and chloride, though in some instances, sulphate may be the sole constituent. The degree of mineralisation varies from 2 to 10g/l. As of 1 January 2016, the commercial reserves of the salt deposit were estimated to be 1,349,600 tonnes or 811000 m3 [22].
2. MATERIALS AND METHODS
Barium sulphate turbidimetry method was used for the determination of sulphate ions, titration with EDTA was used for the determination of calcium and magnesium ions. The sample was subjected to chemical analysis in accordance with the standards set forth in GOST for sodium chloride [23]. An elemental analysis was conducted using neutron-dispersive X-ray fluorescence spectroscopy on an energy-dispersive microanalysis system (INCA Energy 450) mounted on a scanning electron microscope (JSM 6610 LV, JEOL, Japan) and an atomic absorption spectrometer (Kavnt-2). The margin of error is 0.01%. A scanning electron microscope (JSM 6610 LV, JEOL, Japan) was employed to examine the microstructure of the samples. The accelerating voltage was set to 20kV. The imaging mode employed was that of secondary electrons. For analyses in the infrared spectrum, a ShimadzuIRPrestige-21 FTIR spectrometer with the Miracle disturbed total internal reflection (TIR) attachment from PikeTechnologies was used.
3. EXPERIMENTAL
In order to purify the brine from impurities using the phosphate method, a saturated salt solution with a concentration of 315g/dm3 was prepared. The solution was prepared at a temperature of 80-100°C to ensure complete dissolution of the salt. A stoichiometric quantity of sodium phosphate was then added to the saturated solution in order to precipitate the calcium and magnesium salts. The resulting mixture was stirred continuously in a thermostat for a period of 25 to 30 minutes, after which it was left at room temperature for a further 30 minutes and then filtered. The filter retained insoluble salt residues and insoluble calcium and magnesium compounds in a precipitate. The concentration of calcium and magnesium ions in the filtered brine was determined by titration. The solution of sodium chloride (NaCl) is then employed for the treatment and drying of the target product.
4. RESULTS AND DISCUSSION
The natural salt mineral of the Bakhyt-Tany deposit in the Sozak district of the South Kazakhstan region was selected as the subject of investigation. The natural sodium salt mineral in the selected deposit is distinguished by a minimal quantity of impurities and its occurrence at the surface level. The outcomes of the XRD analysis, chemical analysis and elemental analysis (Table 1) of the sodium salt from the Bakhyt-Tany deposit are presented in the preceding paper by Urazkeldiyeva D.A. et al. “Methods for purifying table salt from the Bakhyt-Tany deposit” (Tables 1, 2, Figs. 1-4) [24].
| Element | O | Na | Mg | S | Cl | Ca | Total |
|---|---|---|---|---|---|---|---|
| Weight% | 7,22 | 34,34 | 0,80 | 0,57 | 56,11 | 0,95 | 100,00 |
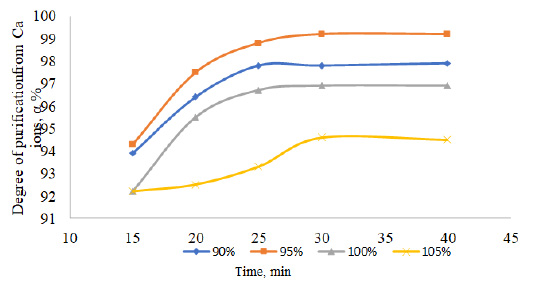
Dependence of the degree of purification of table salt from Ca2+ ions on the duration of the process.
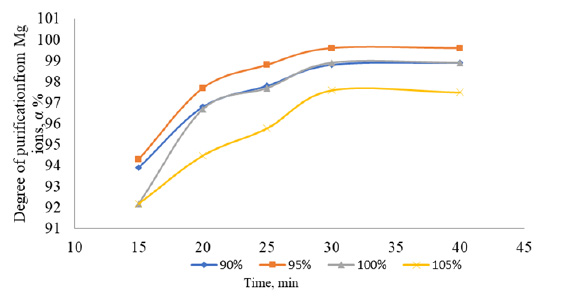
Dependence of the degree of purification of table salt from magnesium ions on the duration of the process.
| The Content of Ions in % | The Composition of the Salt Before Desalination | The Composition of the Salt After Desalination |
|---|---|---|
| Mg 2+ | 0,2857 | 0,2857 |
| Ca 2+ | 0,8807 | 0,8807 |
| Cl - | 53,42 | 53,42 |
| SO42- | 1,83 | 1,83 |
| Na + | 35,3 | 35,3 |
| i.r. | 3.3 | 0,9 |
The remaining salts present in the sodium mineral salt have a very low content, below 2%, and are therefore not visible in the X-ray.
The data indicated that the natural sodium salt derived from the Bakhyt Tany deposit exhibits a high concentration of sodium chloride and a minimal quantity of impurities. In light of the aforementioned data, it can be posited that the natural sodium salt procured from the Bakhyt Tany deposit may be employed as a primary ingredient in the manufacture of table salt and soda ash. The calcium sulphate and other silicate compounds present in the insoluble precipitate can be employed in the production of building materials.
All data, including X-ray fluorescence (XRF), elemental analysis, and chemical analysis, indicate a markedly elevated sodium chloride content in the natural sodium mineral of the Bakhyt-Tany deposit.
Given the mineral's composition is similar to that of table salt, the physicochemical properties were studied in order to obtain sodium chloride, which is used in food and soda ash production. This was achieved by removing calcium and magnesium ions, as well as mechanical additives, from the mineral.
Following the determination of the physico-chemical properties of Bakyt-Tany salt, an investigation was conducted into the desliming process. The desliming process of salt enables the reduction of the quantity of insoluble residue, which has a beneficial impact on the subsequent purification of salt from ions.
The desliming process was conducted using a series of ratios, specifically 1:1, 2:1, 3:1, 4:1, and 5:1 for the L:S ratio. The optimal desliming parameter was determined to be L:S equal to 3:1. A ratio of liquid to solid below 3:1 prevents the passage of insoluble waste and soil-derived waste from natural salt into the liquid phase and their subsequent removal from the crystal formation. In the absence of any alteration to the washing process with a liquid-solid ratio exceeding 3:1, there is no requirement for an increase in the quantity of the liquid phase. Accordingly, the ratio of liquid to solid during flushing was established as 3:1 for saline soils. The results of the chemical analysis of the salt composition, both prior to and following the desalination process, are presented in Table 2.
The insoluble salt residue exhibits two distinct phases. The initial phase is an insoluble clay mass, while the subsequent phase comprises transparent crystals of insoluble calcium sulfate [25].
Following the desalination of the salt, the chemical composition was determined and the most appropriate purification method was selected. A number of salt purification methods designed to remove impurities and ions were selected on the basis of an analysis of the relevant literature. The article presents a discussion of the phosphate method of salt purification from calcium and magnesium ions. The purity of the salt and reagents is of paramount importance in the context of production.
The phosphate purification method is particularly advantageous as it results in the deposition of calcium and magnesium phosphates in the solution, which can then be used in the production of fertilisers. The following reactions are observed during the deposition process Eqs. 1-4:
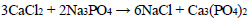 |
(1) |
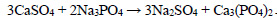 |
(2) |
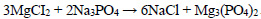 |
(3) |
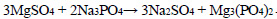 |
(4) |
The washed salt after desalination is dissolved with water until a saturated solution is obtained, while mixing with sodium phosphate in a stoichiometric amount necessary for complete precipitation of dissolved impurities. The study time is 15-40 minutes. The content of sodium phosphate was studied in the range of 90-100% of the stoichiometric ratio. The effective parameter of the sodium phosphate content is 95% of the deposition time is 30 minutes. When using only the phosphate method to purify the solution, the degree of purification of calcium from magnesium ions increased to 97-99%.
The results of the salt purification process, as they pertain to the removal of calcium and magnesium ions, are illustrated in Figs. (1 and 2). In order to ascertain the optimal stoichiometric quantity of sodium phosphate to be employed, the degree of purification at 90, 95, 100, and 105% stoichiometry was evaluated. The addition of sodium phosphate in a 1:1 ratio or greater (i.e., 100% or 105%) results in the presence of 0.1-0.3% phosphate ions in the purified solution, accompanied by a discernible decline in the degree of purification of calcium and magnesium ions. The optimal mixing time is 25 to 30 minutes.
An IR spectral analysis of the precipitated residue was performed during salt purification. The IR analysis of the sediment is shown in Fig. (3).
IR spectroscopic analysis of the percipitate formed from the salt filtrate after the addition of sodium phosphate revealed a distinct absorption band in the region of 1000–1026 cm-1, indicating the presence of a calcium phosphate (Ca–P–O) bond. Single aspects in the 509-589 cm-1 region are characterized by the mineral component of calcium phosphate in the form of Si-O-P Si-O-Mg valence bonds.
The purified saline solution was steamed and dried. A chemical and SEM analysis of the resulting target product was performed. The results of the SEM analysis showed that the product contains 36.87% of the mass fraction of sodium and 63.13% of chlorine.
4.1. Technological Scheme
According to the obtained results of the research the technological scheme of purification of Bakhyt-Tan deposit from impurities of sodium chloride was developed. Fig. (4) shows the technological scheme of table salt purification.
Table salt from the lake is loaded into the mixer (1) and washed from mechanical impurities by circulating saturated solution at a temperature 20-25°C for 30 minutes at mass ratio solution:salt=3:1. The obtained suspension is separated by screening in the (2), the solution with mechanical impurities is separated on the nutch-filter (3), the solution is returned to sodium chloride salt washing. The washed ore is dissolved with water and washing water from the filter press to saturation in the reactor (4) at a temperature of 25°C, then sodium phosphate is added to the solution in an amount of 95% of stoichiometry to p is characterized by precipitate impurities of calcium and magnesium. The obtained suspension is sedimented in a settling tank (5), and the thickened pulp with impurities is washed on a filter press (6). The water-washed and squeezed sludge is sent for disposal, and filtrate and washing water are returned for mixing and salt dissolution in the reactor (4).
The clarified part of the solution is drained by decantation from the settling tank and transferred to the spray dryer (7) for drying with flue gas at a temperature of 100-110°C with crystallisation of sodium chloride. The dried crystallised product from the dryer is transferred to the screw feeder (9) and, after cooling, further onto the packing. Dust with flue gas is cleaned on cyclones (8) and after cleaning is discharged into the atmosphere.
The advantage of the proposed method is the exclusion of concentrated hydrochloric acid from the purification process, leading to corrosion of equipment and the need for thorough washing of sodium chloride crystals with high-purity water, as well as simplification of the technological process.
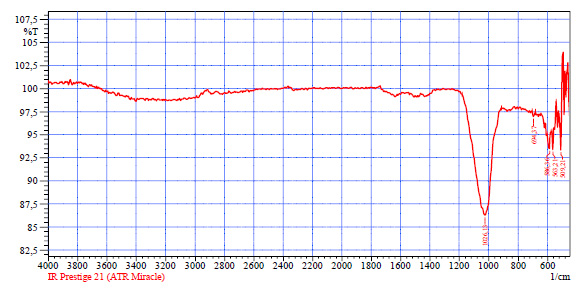
IR analysis of the sediment obtained by precipitation of ions by the phosphate method.
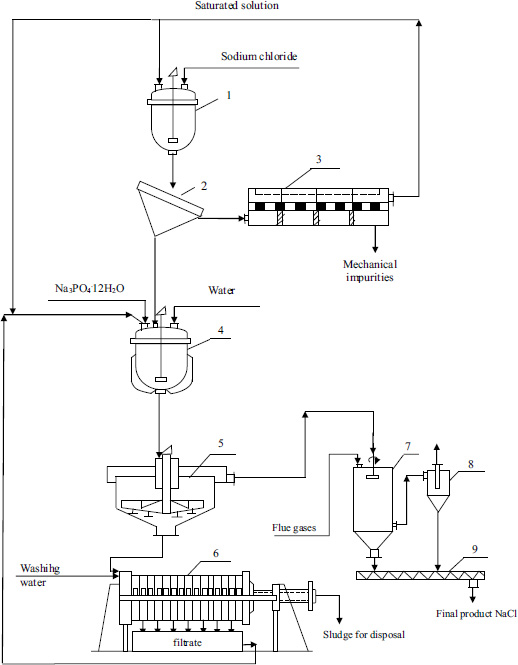
Тechnological scheme of table salt purification
CONCLUSION
The results of the study demonstrate that the natural mineral salt of the Bakhyt-Tany deposit is characterised by a high concentration of sodium chloride (88.4%) and contains a limited number of impurities, including calcium sulphate (2.5%), magnesium sulphate (0.18%), magnesium chloride (0.37%) and insoluble residue (up to 2% by mass). The high NaCl content of the mineral renders it a promising raw material for industrial use; however, the presence of impurities necessitates the implementation of effective purification methods.
Analysis by X-ray, elemental and chemical methods confirmed that the chemical composition of the natural raw material corresponds to previously published data and demonstrated the necessity of desliming before using the mineral in the food industry. It was established that the optimal ratio of liquid and solid phases in the desliming process maximises the removal of insoluble residue, thereby enhancing the filtration and technological characteristics of the solution. During the course of the experiments, it was determined that the utilisation of sodium phosphate in the stoichiometric ratio of 95-100% enables the attainment of a high degree of NaCl purification from magnesium and calcium ions, achieving an efficiency of 99%. This finding underscores the viability of the method for industrial implementation, particularly in the production of high-purity NaCl, a critical component in the chemical, food, and pharmaceutical sectors.
The devised technological scheme for sodium chloride purification by the phosphate method enables the minimization of undesirable impurities, thereby ensuring cost-effectiveness and efficiency in the production process.
AUTHORS’ CONTRIBUTIONS
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| XRD | = X-ray diffraction |
| IR | = Infrared spectroscopy |
| TIR | = Total internal reflection |
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author upon request.
FUNDING
Intramural grant project UKU2024-005 was provided by South Kazakhstan Research University “Zhas Galym”.
ACKNOWLEDGEMENTS
The authors express their gratitude to the intramural grant project UKU2024-005 of South Kazakhstan Research University “Zhas Galym” for financial support and contribution to this research.


