All published articles of this journal are available on ScienceDirect.
Obtaining Polyaromatic Raw Materials for Needle Coke By Solvolysis Of Russian And Mongolian Coals using Coal- And Petroleum-derived Heavy Residues as Solvents
Abstract
Introduction
The purpose of this research is to obtain polyaromatic substances via solvolysis of Mongolian and Russian coals and their characterization as raw materials for needle coke preparation.
Methods
The quinoline-soluble products were obtained via coal solvolysis at a moderate temperature of 380°C using heavy hydrocarbon fractions of coal and petroleum origin as solvents.
The chemical and group composition and molecular structure of the soluble products were characterized by FTIR, GC-MS, gas and liquid phase chromatography, and TG-DTG-DSC techniques.
Results
The products obtained from the coal solvolysis were pitch-like matter soluble in quinoline up to 90-95%. The main components were represented predominantly by polycondensed aromatics, and their structures depended on the coal and solvent used. A remarkable feature of the polyaromatic products was a reduced concentration of carcinogenic benzo(a)pyrene (up to 40 times compared to commercial coal-tar pitch).
Discussion
The product obtained with coal tar as solvent was highly aromatic, and its aromatic nuclei consisted predominantly of polycondensed sparsely substituted cycles. The product obtained with petroleum-derived solvent was less aromatic, and the aromatic units were highly alkylated with fairly long alkyl chains. The pitch-like product with the intermediate structural parameters was obtained using a binary solvent.
Conclusion
In terms of composition and molecular structure, the pitch-like products obtained via coal solvolysis can serve as a new polyaromatic feedstock with reduced carcinogenicity for the preparation of valuable carbon materials. By selecting solvents and coals, it is possible to optimize the molecular-structural characteristics of the products in order to obtain feedstock for the production of high-tech carbon materials, including needle coke and valuable chemicals.
1. INTRODUCTION
Coal is currently primarily used as a fuel for electric energy and heat generation via combustion, which results in a large carbon footprint. However, the world’s innovative experience in the development of the coal industry has already proved wide possibilities and prospects for using coal also for the production of specialty chemicals and carbon-based materials with a high added value, like carbon electrodes, needle coke, carbon fibers, activated carbon, graphite materials of various applications [1, 2].
Mongolia and Russia have large reserves of various coals [3, 4]. In Mongolia, they are used mainly for energy generation through direct combustion. In Russia, in addition to energy generation, a large amount of bituminous coal is processed through high-temperature chamber coking to produce metallurgical coke for blast furnace technology of steel making. High-temperature coal tar is obtained simultaneously as a minor by-product, which is used for the production of chemicals and carbon materials, particularly pitch binders for carbon anodes.
The key feature that makes coal tar attractive for the production of many carbon materials is the polycondensed aromatic nature of its molecules, which are the building blocks in the structure of carbon materials [5]. Among them, one of the most important is needle coke, which is recognized as an indispensable material in both modern strategic industries, such as metallurgy (steel smelting in electric arc furnaces with ultra-high power graphite electrodes and aluminum electrolysis with carbon anodes) as well as in new emerging energy sectors such as lithium-ion batteries and supercapacitors [5-7].
The production of needle coke requires specific raw materials with a low content of heteroatoms and other impurities, a high content of aromatics with polycondensed rings, and a moderate alkyl substitution. The main feedstocks for needle coke production are based on the refined fractions obtained from coal tar and heavy petroleum residues [5]. Meanwhile, a steady decline in the demand for metallurgical coke has been observed in the last decade, which results in a gradual decline (apparently irreversible) in its production and, as a consequence, in the production of coal tar [8, 9]. The resources of high-quality petroleum feedstock are also limited because the global petroleum industry is characterized by a steady increase in the share of heavy high-sulfur oils [10, 11]. When using such feedstock, complex preliminary preparation processes are necessary, including aromatization, deasphalting, demetallization, purification from heteroatoms, and other undesirable components, which result in increased cost of the final product [11, 12]. The factors noted are growing concerns about the availability of polyaromatics for the carbon industry, which stimulates the search for new sources for the production of needle coke and other valuable carbon materials.
An alternative reliable and prospective method for producing polyaromatic compounds is low-temperature dissolution of coals instead of high-temperature coking [13-18]. Compared to petroleum, coal is richer in polycondensed aromatics which are fairly close to the components of coal tar. The coal dissolution process has attracted much attention in the last two decades. The most active developments are being carried out in Japan, China, and the USA. Kobe Steel Co. Ltd. and Mitsubishi Chemical Co. in Japan are jointly developing a low-temperature solvent process for producing a pitch-like Hypercoal product, which is a concentrate of polycondensed aromatics [19, 20]. It has been shown that Hypercoal can be used for the production of various carbon materials, such as high-quality coke, carbon binder, and an additive to the coking batch. Researchers at the University of Kentucky, Pennsylvania State University, and West Virginia State University are developing a coal dissolution process to obtain pitch-like products as raw materials for obtaining various carbon materials [21, 22].
Coal has large reserves in many countries. The implementation of the industrial processes for its conversion into soluble polyaromatics will satisfy the growing needs of strategically important industries for chemicals and high-tech carbon materials: needle coke, carbon fiber, carbon electrodes for new emerging energy sectors, and other carbon and composite materials. The production of polyaromatics via thermal dissolution of coal has significant advantages over traditional coal processing via hydrogenation, coking, and semi-coking since it is carried out under mild conditions (moderate temperature, low pressure) without the use of hydrogen and catalysts.
We recently studied [23-25] the dissolution of coals at temperatures of 350-380oC under liquid-phase conditions using technical coal- and petroleum-derived heavy hydrocarbon fractions as solvents. This process is considered a method of co-processing coals with heavy hydrocarbon residues into valuable products. Based on data on the composition of the initial coal-solvent slurry and resulting reaction products, the process of coal dissolution at low temperatures of 350-380oC is considered to occur due to solvent-induced thermal depolymerization of coal matter along the weakest linkages between the polycondensed aromatic rings, rather than solely due to thermal bond cleavage [23]. The highest yield of quinoline-soluble substances was achieved for bituminous coals in the temperature range of 350-380°C when coals passed into a plastic state. The correlation analysis was performed, and the coal-related parameters were determined, ensuring effective conversion into polyaromatic quinoline-soluble products at 380ºC [24].
The goal of this study was to obtain quinoline-soluble products via low-temperature dissolution of Russian and Mongolian coals in various high-boiling solvents of coal and petroleum origin and to characterize the chemical and group composition and properties of the obtained products from the point of view of raw materials for the production of chemicals and needle coke. The molecular structure of the main dissolution products was characterized through IR-Fourier spectroscopy in combination with detailed GC-MS and liquid-phase chromatographic analysis of some narrow fractions isolated from the dissolved products.
2. MATERIALS AND METHODS
2.1. Coal and Solvents Used
Four bituminous coals were used for dissolution: two samples from the basins in Russia (Chadan deposit in Ulugkhemsky Basin, CR coal, and Kuzbass basin, KR coal) and two samples from the Tavantolgoi (TM coal) and Nariinsuhait (NM coal) deposits in Mongolia. The coal samples were ground to a fraction of < 1 mm and dried in a vacuum oven at 85°C before use.
The commercially available Coal Tar (CT), its Anthracene Oil (AO), Heavy Gas Oil (HGO) derived from the catalytic cracking of petroleum fraction, and a binary CT+HGO blend were used as solvents for coal dissolution.
Typical commercial coal-tar pitches and petroleum-derived pitches were used as reference samples with softening points of 88°C and 89°C, respectively.
2.2. Reactor Unit and Dissolution Procedure
The coal dissolution reaction was carried out following previously optimized conditions using an experimental unit equipped with a 2 l stainless steel autoclave with a mechanical stirrer [23, 24]. The autoclave was charged with coal/solvent slurry (900 grams to 1260 grams, with the proportion of coal to solvent of 1:2 by the weight), hermetically sealed, and purged carefully with nitrogen. The reaction was carried out at 380°C for 1 hour (or 3 hours) at autogenous pressure.
At reaction completion, the autoclave was allowed to cool to 250°C and then depressurized. The vapor-gas products were vented through a refrigerator line, while the gases and condensed liquid were collected and measured. Subsequently, the valve at the bottom of the autoclave was opened, and the molten digested product (which consisted of dissolved coal in a solvent + ash coal residue) was drained off into a cylinder receiver. Following that, it was allowed to cool while stirring for homogenization and then pushed out of the cylinder through a piston. The digested pitch-like product was packaged and stored in a plastic bag, and the gaseous products were analyzed via gas chromatography.
The representative portion of the pitch-like product (about 20 grams) was dispersed under ambient conditions, and 2 grams of sample was taken for analysis. Soxhlet extraction was used to determine the toluene-soluble fraction. Toluene insoluble residue was dried under vacuum at 80°C, weighed, and further extracted with a hot quinoline until the solution became clear. Quinoline insoluble residue was washed with toluene, dried under vacuum at 80 °C, and weighed. The analysis was repeated two to three times to confirm the reproducibility.
2.3. Analytical Techniques
The contents of moisture, ash, volatile matter, and vitrinite reflectance were determined using standard procedures. The ultimate analysis was performed using a FLASHTM 1112 analyzer. Moreover, the volatility of the solvents was determined through the atmospheric distillation.
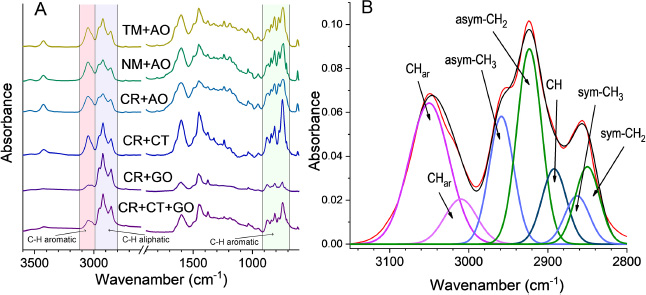
Coal conversion was determined based on the ash content in the initial coal sample (Ac) and the quinoline– insoluble residue (Ar) using the following Eq. (1):
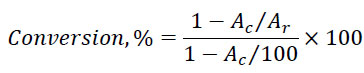 |
(1) |
Two to three repeated coal dissolution experiments were carried out, and the variations in the conversion of coal were within 1.5%.
The FTIR spectra were recorded from the KBr pellets using a Tensor-27 FTIR spectrometer within the spectral range from 4000 to 400 cm-1.
TSQ 8000 Trace 1310 Gas Chromatography-Mass spectrometer was used for identifying and quantifying organic compounds present in the heptane soluble and distilled fractions. Capillary column TR-5ms 60 m × 0.25 mm × 0.25 μm was employed. Soxhlet extraction was used to isolate the heptane soluble fractions for GC–MS analysis.
The concentration of benzo(a)pyrene (BaP) in the solvents and the toluene-soluble fractions of the products was measured using an LC20 high-performance liquid chromatograph.
The gaseous products were analyzed using a Crystallux–4000M gas chromatograph equipped with two columns and thermal conductivity detectors. The program NetCrom v2.1 was used for chromatographic data treatment.
Thermogravimetric Analysis (TGA) was carried out using a STA 449 F1 Netzsch Jupiter instrument in a temperature range of 30°C to 1000°C in an argon flow.
The viscoelastic properties of the pitch-like products were characterized by softening points using a “ring-and-ball” method according to ASTM: D36/D36M–14. The softening point corresponded to the temperature at which a metal ball passed through a ring filled with the softened pitch.
3. RESULTS AND DISCUSSION
3.1. Characterization of Used Coals and Solvents
The data in Table 1 show the composition and properties of the coals used. They correspond to bituminous (gaseous fat and fat grades) of vitrinite type with the vitrinite reflectance Ro,r, of 0.77% to 1.12 and volatile yield ranging from 36.1 to 27.7 wt.%.
The data in Table 2 show that the petroleum-derived HGO solvent contains more hydrogen and fewer heteroatoms compared to CT and AO solvents. All solvents began to distill off at a temperature above 170-221°C. The distillation maximum was observed at around 300-350°C.
3.2. Coal Dissolution
3.2.1. Dissolution Products and their Characterization
Table 3 shows examples of the mass balance on the dissolution experiments using Chadan Coal (CR) and various solvents at 380°C for 1 hour. Autogenous pressure ranged from 1.4 to 2.5 MPa depending on the solvent. The main reaction product obtained was pitch-like matter consisting of dissolved coal + solvent + ash coal residue. Small amounts of gases (0.4-0.6% wt.%) and condensed naphtha (0.55 to 4.80%), which were collected when the autoclave was depressurized at 250°C, were formed during 1 hour of reaction. Increasing the dissolution duration to 3 hours led to an increased gas formation (from 0.60 to 1.3%). The mass balance, calculated as the total yields of the pitch-like product + naphtha + gas, was no less than 96.7%. Losses due to the difficulty of quantitatively extracting the viscous product from the autoclave, receiver, and shut-off equipment amounted to 1.6-3.3%.
The data in Table 4 show that the gaseous products consist mainly of CO2 (much less CO), CH4, C2-C4 hydrocarbons, and hydrogen. The proportion between the yields of gases depended on the solvents used, and the enhanced amounts of methane and C2-C4 gases and less hydrogen were observed when petroleum-derived HGO solvent was used. An increased amount of H2S was obtained with coal-derived CT and AO solvents.
| Coal Deposit | Coal Grade | wt.% | Ultimate Analysis, wt.% on daf | Ro,r, % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Аd | Vdaf | С | Н | N | S | О* | |||
| Chadan (CR) | Gaseous fat | 5.6 | 35.8 | 84.7 | 5.5 | 1.3 | 0.6 | 7.9 | 0.77 |
| Kuzbas (KR) | Fat | 11.2 | 34.5 | 87.5 | 5.6 | 0.9 | 0.5 | 5.5 | 0.93 |
| Nariinsukhait (NM) | Gaseous fat | 6.8 | 36.1 | 84.4 | 4.8 | 1.6 | 1.0 | 8.2 | 0.74 |
| Tavantolgoi (TM) | Fat | 8.2 | 27.7 | 87.8 | 5.2 | 1.2 | 0.4 | 5.4 | 1.12 |
| Solvent | Element Composition, wt.% | H/C at. | Distillation Temperature Range, °С |
||
|---|---|---|---|---|---|
| С | Н | N+S+O | |||
| AO | 88.7 | 5.3 | 5.7 | 0.72 | 170-350 |
| CT | 91.5 | 5.3 | 3.2 | 0.69 | 180-550 |
| HGO | 89.9 | 8.3 | 1.8 | 1.11 | 221-508 |
| Solvent Used | Autoclave Loading with Coal-solvent Slurry, g | Products, wt.% | Loss. % | ||
|---|---|---|---|---|---|
| Pitch-like product |
Gas | Naphtha | |||
| CT | 1260 | 93.2 | 0.40 | 4.80 | 1.6 |
| HGO | 1260 | 96.8 | 0.52 | 0.55 | 2.1 |
| CT+HGO* | 1200 | 94.6 | 0.60 | 1.70 | 3.1 |
| CT+HGO*, 3 h | 1200 | 93.8 | 1.30 | 1.70 | 3.3 |
| Solvent | CO2+CO | CH4 | H2 | C2-C4 | H2S |
|---|---|---|---|---|---|
| AO | 44.3 | 22.4 | 17.2 | 15.2 | 0.9 |
| CT | 36.2 | 25.1 | 19.6 | 16.9 | 2.0 |
| HGO | 25.3 | 40.2 | 9.8 | 24.2 | 0.5 |
| CT+HGO | 29.8 | 32.5 | 14.7 | 21.5 | 1.5 |
Fig. (1) shows the thermogravimetric TG, DTG, and DSC curves reflecting the thermal behavior of the pitch-like products obtained by dissolving coals using AO solvent. The yield of volatile substances ranges from 63% to 68%, and the maximum rate of volatile release is observed at 300-320°C. The DSC curves show that the thermal transformations are accompanied by a series of endothermic effects. Low-temperature endoeffects with maxima at temperatures of about 80°C and 290-330°C were caused by the evaporation of light components. At temperatures above 600°C, thermal transformations were accompanied by significant endothermic effects with small changes in the mass of the samples. These high-temperature effects may be associated mainly with structural transformations of the solid chars: in a series of pitch-like samples obtained with CR, NM, and TM coals, the endothermic peaks shift towards higher temperatures from 620°C to 670°C and 760°C, respectively.
Figs. (2A, B) shows the results of the atmospheric distillation of the pitch-like products when heated to 350°C. The yields of the liquid fractions ranged from 14.7% to 29.0%, and pitch residues from 71.0% to 85.3%, depending on the solvent and coal used. The increased yields of liquid fraction (27.5-29.0%) were obtained when CR and NR coals of lower rank were dissolved in the AO solvent.
Fractional distillation showed (Table 5) that the liquids from the pitch-like product obtained using CT solvent consisted mainly of anthracene and creosote oils (51.0% and 35.6%, respectively) with smaller amounts of carbolic and naphthalene ones. The anthracene fraction predominated (75.6% to 87.5%) in the distillates from the products obtained by the dissolution of various coals in other solvents.
3.2.2. Characterization of the Chemical Composition and Properties of the Pitch-like Products
The conversion of coals, the group composition of the products, and their softening points are shown in Table 6. The product obtained using AO, CT, and binary solvent represented typical pitch-like matter with softening points of 80 to 90°C. The product obtained with HGO solvent was soft, viscous matter without a certain softening point.
The conversion of coals for 1 hour of reaction ranged from 76.0% to 82.5%. The solvent performance for CR coal dissolution decreased in the following order: AO ≈ CT+HGO > HGO > CT. The petroleum-derived HGO solvent was almost as effective as the coal-derived CT solvent, and their binary blend showed some synergistic
TGA analysis of the pitch-like products obtained on the dissolution of different coals in AO at 380°C.

The result of the atmospheric distillation of the pitch-like products when heated to 350°C. (A) CR coal and different solvents and (B) AO solvent and different coals.
| Liquid Product (Coal+solvent used) |
Yield, % | |||
|---|---|---|---|---|
| Carbolic oil (170-210 °С) |
Naphthalene oil (210-230 °С) |
Creosote oil (230-300 °С) |
Anthracene oil (300-350 °С) |
|
| CR+CT | 1.8 | 11.6 | 35.6 | 51.0 |
| CR+HGO | 1.4 | 1.1 | 13.4 | 84.1 |
| CR+CT+HGO | 1.1 | 4.9 | 18.4 | 75.6 |
| CR+AO | 1.4 | 1.6 | 14.0 | 83.0 |
| NM+AO | 2.2 | 2.0 | 17.2 | 78.6 |
| TM+AO | 2.1 | 1.1 | 9.3 | 87.5 |
| Solvent+coal | Coal Conversion, % |
Content of Solubles, wt.% on daf |
Quinoline Insoluble, % | Softening point, °C |
|
|---|---|---|---|---|---|
| In toluene | In quinoline | ||||
| AO+CR | 82.5 | 74.0 | 92.9 | 7.1 | 80 |
| AO+NM | 81.8 | 69.7 | 92.4 | 7.6 | 89 |
| AO+TM | 76.0 | 71.0 | 90.2 | 9.8 | 82 |
| CT+CR | 77.0 | 64.4 | 91.8 | 8.2 | 86 |
| HGO+CR | 78.0 | 77.6 | 92.1 | 7.9 | - |
| CT+HGO+CR | 82.0 | 73.0 | 92.9 | 7.1 | 82 |
| CT+HGO+CR. 3h* | 79.0 | 71.0 | 90.8 | 9.2 | 90 |
| Commercial coal-tar pitch | - | 64.9 | 89.5 | 10.5 | 88 |
| Commercial petroleum-derived pitch | - | 74.1 | 99.6 | 0.4 | 89 |
effect, resulting in higher coal conversion and the smallest content of the quinoline insoluble (7.1% compared to 8.2% and 7.9% in the case of each solvent separately).
The group composition of the pitch-like products consisted of 64.4% to 77.6% of toluene solubles and 90.2% to 92.9% of quinoline solubles. The product obtained using the CT solvent had a reduced proportion of toluene-soluble substances (64.4%), whereas the product obtained using the HGO solvent was enriched in these solubles (77.6%).
The results of the ultimate analysis of the pitch-like products are shown in Table 7. It can be observed that the product obtained with HGO solvent is enriched in carbon and hydrogen (90.3% and 7.4%, respectively) and depleted with oxygen (0.8%) compared to products obtained with coal-derived solvents. The concentrations of sulfur ranged from 0.5% to 1.4%, depending on the solvent and coal.
The composition of the petroleum-derived pitch was within the composition range of the pitch-like products obtained, and commercial coal-tar pitch showed most higher atomic C/H ratio compared to others.
3.2.3. Characterization of the Molecular Structure of the Pitch-like Products
The high complexity of the composition of pitch-like products predetermines the need to evaluate the average structural characteristics of organic matter using spectroscopic techniques in combination with the detailed analysis of the separated soluble and distilled fractions of a narrower composition using chromatographic methods.
3.2.3.1. FTIR Analysis
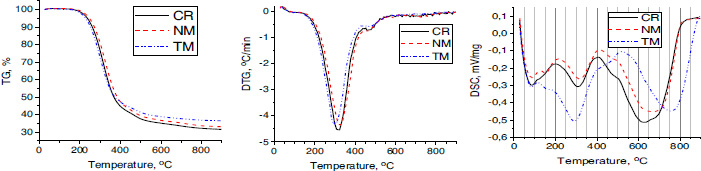
Figs. (3A, B) shows the FTIR spectra, which reflect the variation of the molecular structure of the pitch-like samples. The absorption bands centered at 1600 cm-1 (stretching vibrations of the aromatic C-C bonds), 3045 cm-1 (stretching vibrations of the aromatic C-H bonds), and 900-700 cm-1 (out-of-plane bending of the aromatic C-H bonds) indicate the aromatic structures. HGO product has less intensity in these spectral regions reflecting lower aromaticity compared to other products. The broad absorbances in the spectral ranges of 3000-2750 cm-1 (stretching vibrations of the aliphatic C-H bonds) and 1460-1370 cm-1 (bending vibrations of the aliphatic C-H bonds) are characteristic of the aliphatic groups of different configurations. Very small absorbance at nearly 3430 cm-1 can indicate small amounts of phenol hydroxyls as well as nitrogen-containing heterocycles with the N-H bond (like in indole and carbazole). Barely noticeable shoulders at nearly 1750 cm-1 and 1650 cm-1 reflect small amounts of carbonyl and carboxyl groups.
Absorption in the characteristic spectral regions of 3100-3000 cm-1, 3000-2750 cm-1, and 900-700 cm-1 were deconvoluted according to well-founded guidelines [26-28], and the corresponding semiquantitative molecular indexes were estimated. Other spectral regions also show
| Coal | Solvent | Content, wt.% based on daf | C/H atomic ratio | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | О | |||
| CR | AO | 89.2 | 5.3 | 1.4 | 0.6 | 3.5 | 1.40 |
| NM | AO | 88.7 | 5.3 | 2.1 | 0.5 | 3.4 | 1.39 |
| TM | AO | 89.5 | 5.2 | 1.4 | 0.6 | 3.3 | 1.43 |
| CR | CT | 89.7 | 5.4 | 1.3 | 1.0 | 2.6 | 1.38 |
| CR | HGO | 90.3 | 7.4 | 0.6 | 0.9 | 0.8 | 1.02 |
| CR | CT+HGO | 88.7 | 6.6 | 1.1 | 1.3 | 2.3 | 1.12 |
| CR | CT+HGO, 3 h* | 89.7 | 5.9 | 1.2 | 1.4 | 1.8 | 1.27 |
| Commercial coal-tar pitch | - | 92.5 | 4.6 | 1.1 | 0.6 | 1.2 | 1.68 |
| Commercial petroleum-derived pitch | - | 91.7 | 5.7 | 0.8 | 1.3 | 0.5 | 1.34 |
FTIR spectra of the products obtained via dissolution of coals using different solvents and coals. (A) pitch-like products obtained on the dissolution of coals in different solvents; (B) deconvoluted FTIR spectra from the product obtained via dissolution of TM coal using AO solvent.
variations in the absorption; however, they are less characteristic. For instance, the spectra show a large variation in the intensity of the absorption centered at 1600 cm-1 related to the stretching vibration of the aromatic C-C bonds. However, the extinction coefficient for this mode of vibration depends greatly on the structure of the aromatic unit, type of substituents, and degree of substitution, and, therefore, this band can hardly be used for semiquantitative estimation.
The characteristic absorption in the 3100-2750 cm-1 region was best simulated by seven Gaussian sub-bands, which were assigned to two types of aromatic C-H bonds and five types of aliphatic C-H bonds of different configurations. The absorption in the 900-700 cm-1 region was best fitted to 8-10 sub-bands, which reflect the features of the structure of the aromatic units, i.e., the number of adjacent C-H bonds in the aromatic ring and, consequently, the degree of the aromatic ring substitution (or condensation). A well-defined sub-band centered at 740 cm-1 (four adjacent C-H bonds) was assigned to ortho-substituted (low-substituted) aromatic rings [26].
The following molecular indexes were estimated based on the areas of the characteristic sub-bands: Har hydrogen aromaticity index, Car carbon aromaticity index, Iort index for ortho-substituted aromatic rings, and proportion between the number of C-H bonds in the CH3 and CH2 groups, which reflects the structure of the aliphatic fragments (the length or degree of branching). Semi-quantitative molecular indexes were calculated taking into account the corresponding extinction coefficients [27, 28] using the following formulas 1-4:
 |
(1) |
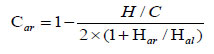 |
(2) |
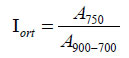 |
(3) |
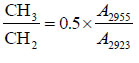 |
(4) |
Table 8 shows the FTIR indexes thus estimated. It is evident that the indexes reflecting the molecular structure of the products depend on both the coal and the solvent used. The products obtained with CT and AO solvents have high aromatic structures (Car=0.87-0.92 and Har=0.67-0.77). The product obtained using HGO solvent is the least aromatic (Car=0.64 and Har=0.31), with the aromatic rings being highly substituted (Iort=0.20).
The product obtained with binary solvent shows molecular indexes that are almost intermediate compared to those obtained with each solvent separately. An increase in dissolution duration to 3 hours increases the aromaticity and CH3/CH2 ratio, indicating the occurrence of aromatization reaction and cleavage of C-C bonds in the aliphatic substituents. The products obtained from different coals have almost similar aromaticity indexes but differ in Iort value. The lowest Iort value of 0.20 for the product obtained from the NM coal shows the high extent of aromatic ring substitution.
3.2.3.2. GC-MS Analysis
The detailed composition of the fractions isolated from the pitch-like products via heptane extraction was studied using GC-MS. Fig. (4) shows the GC-MS diagrams of the heptane-soluble fractions from the pitch-like products obtained via dissolving +CR coal in CT, HGO, and CT+HGO blended solvents, and Table 9 displays the concentration of the main compounds identified. The data show that polycyclic aromatic hydrocarbons with 2-4 benzene rings are the dominant class of compounds. The highest concentration of this group was detected in the heptane fraction separated from the pitch-like product obtained via CR coal dissolution in the CT solvent. The main components are naphthalene, anthracene, phenanthrene, pyrene, and their homologs. Anthracene and phenanthrene are present in the HGO product, mainly as alkylated homologs, and in the CT product, mainly as naked polycycles.
The hydroaromatic compounds are represented mainly by fluoranthene, acenaphthene, fluorene tetrahydroanthracene and derivates, and heterocyclic compounds mainly by dibenzofurane, benzoquinone, carbazole, and dibenzothiophene derivates. In the HGO product, long-chain n-alkanes ranging from heptadecane to hexacosane were also detected in significant concentrations. The heptane soluble fraction from the CT+HGO pitch-like product is intermediate in its composition compared to those isolated from the products obtained with each CT and HGO solvent. In all samples, the linear structure of the aromatic compounds predominated over angular ones. In total, GC-MS data on the individual composition of the heptane soluble fractions confirm the results of FTIR analysis, which showed an increased content of aliphatic structures, moderate content of aromatic compounds, and a high degree of substitution by alkyl groups in the HGO product compared to CT and AO products.
The light distilled fraction (the boiling point of 210-300 °C) from the pitch-like product obtained via coal dissolution using AO solvent consisted mainly of a mixture of 2-3 rings of aromatic compounds (Table 10). The main compounds identified are aromatic hydrocarbons, like naphthalene, anthracene, phenanthrene, and alkylated derivates. Hydroaromatic compounds, such as acenaphthene, fluorene, and dihydroanthracenes, were also detected. The main heterocyclic compounds were carbazole, dibenzofurane, isoquinoline, benzothiophene, and derivates.
| Coal Used | Solvent Used | Aromaticity Index | Ortho- Substitution, Iort. |
СН3/СН2 Ratio |
|
|---|---|---|---|---|---|
| Сar | Har | ||||
| CR | CT | 0.87 | 0.67 | 0.44 | 0.42 |
| CR | HGO | 0.64 | 0.31 | 0.20 | 0.33 |
| CR | СT + HGO | 0.76 | 0.46 | 0.35 | 0.34 |
| CR | CT+HGO, 3 h* | 0.78 | 0.50 | 0.37 | 0.41 |
| CR | AO | 0.90 | 0.75 | 0.27 | 0.43 |
| KR | AO | 0.91 | 0.77 | 0.44 | 0.34 |
| NM | AO | 0.92 | 0.73 | 0.20 | 0.38 |
| TM | AO | 0.90 | 0.77 | 0.34 | 0.33 |
| Coal-tar pitch | - | 0.94 | 0.89 | 0.37 | 0.23 |
| Petroleum-derived pitch | - | 0.88 | 0.79 | 0.27 | 0.43 |
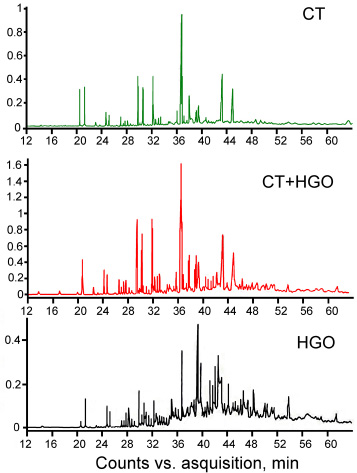
GC-MS diagrams for the heptane soluble fractions separated from the pitch-like products obtained via CR coal dissolution in CT, HGO, and in blended CT+HGO solvents.
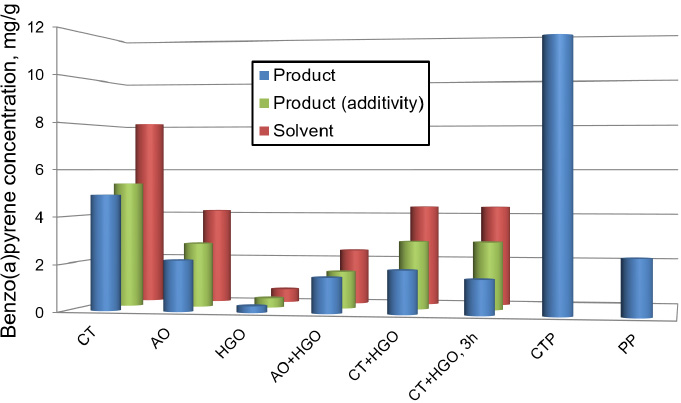
Benzo(a)pyrene concentrations in the solvent and pitch samples.
| Compound | Solvents used for Coal Dissolution | ||
|---|---|---|---|
| CT | CT+HGO | HGO | |
| Tetrahydronaphthalene | 3.36 | 0.12 | 0.21 |
| Quinoline | 0.52 | 0.45 | 0.10 |
| Naphthalene | 3.95 | 1.86 | 1.39 |
| Alkylated naphthalenes | 8.94 | 8.70 | 6.05 |
| Acenaphthene | 0.89 | 0.72 | 0 |
| Dibenzofuran | 3.68 | 3.5 | 1.06 |
| Diphenylenomethane | 5.39 | 5.48 | 1.32 |
| 4-methyl, 1,1-biphenyl | 0.54 | 0.65 | 0.74 |
| 2-hydroxyfluorene | 0.59 | 0.74 | Traces |
| 4-methyldibenzofuran | 0.83 | 1.03 | 0.13 |
| Dibenzothiophene | 1.44 | 1.19 | 0.83 |
| Benzoquinone | 0.82 | 0.67 | Traces |
| 9-methanol-9H-carbazole | 3.54 | 2.75 | 1.24 |
| 3-methyldibenzothiophene | Traces | Traces | 0.75 |
| Anthracene | 19.03 | 18.03 | 4.57 |
| Alkylated anthracenes | 1.79 | 10.40 | 21.0 |
| 1,2,3,4-tetrahydroanthracene | 0.57 | 0.70 | 0.19 |
| Phenanthrene | 4.17 | 3.65 | Traces |
| Alkylated phenanthrenes | 3.83 | 5.85 | 27.35 |
| 2-phenylnaphthalene | 0.62 | 1.05 | Traces |
| Fluoranthene | 12.3 | 9.76 | 1.68 |
| Pyrene | 2.67 | traces | 1.57 |
| 1-(phenylmethyl)-1H-indene | 2.1 | 7.6 | 0.76 |
| 3,4-benzofluorene | 1.31 | 1.27 | Traces |
| 3,4-benzo-thiofluorene | 0.66 | Traces | Traces |
| Benzanthracenes | 5.14 | Traces | Traces |
| Benzophenanthrene | 0.85 | Traces | Traces |
| n-alkanes С17-С26 | - | 2.6 | 14.0 |
| Component | Content, % |
|---|---|
| Octane | 0.2 |
| Para-xylene | 0.4 |
| 1,3-dimethylbenzene | 0.5 |
| Naphthalene | 23.0 |
| Benzothiophene | 0.4 |
| Isoquinoline | 1.8 |
| Quinoline | 1.0 |
| 2-methylnaphthalene | 5.6 |
| 1-methylnaphthalene | 2.4 |
| Biphenyl | 1.6 |
| 2-ethylnaphthalene | 0.5 |
| 2,7-dimethylnaphthalene | 0.8 |
| 1,6-dimethylnaphthalene | 0.7 |
| 2,3-dimethylnaphthalene | 0.4 |
| 1,3-dimethylnaphthalene | 0.4 |
| Acenaphthene | 8.6 |
| 2-naphthalenecarbonitrile | 0.8 |
| Dibenzofuran | 10.0 |
| Fluorene | 13.1 |
| Diphenylmethane | 1.1 |
| 9-methyl-9H-fluorene | 1.0 |
| 2-methyl-1,1´-biphenyl | 0.8 |
| 4-methyldibenzofuran | 1.3 |
| 9,10-dihydroanthracene | 0.6 |
| 6,9-dihydroanthracene | 0.3 |
| 3-methyl-9H-fluorene | 0.4 |
| 1,2,3,4-tetrahydroanthracene | 1.5 |
| Anthracene | 12.6 |
| Phenanthrene | 5.0 |
| 5H-indeno[1,2-b]pyridine | 1.0 |
| 4H-cyclopenta[def]phenanthrene | 0.3 |
| Fluoranthene | 0.5 |
| 4-methyl-1,1´-biphenyl | 0.2 |
| 2-methylbenzofuran | 1.5 |
| 9-methyl-9H-fluorene | 0.2 |
| Carbazole | 3.2 |
| 2,6-dimethylnaphthalene | 0.7 |
| 4-methyl-1,1´biphenyl | 0.6 |
Detailed analysis and identification of the 300-340°C distillate fraction were difficult to quantify because of high-boiling points and complex structure. Among the main polyaromatic hydrocarbons, anthracene (27%), phenanthrene (9.8%), pyrene (1.2%), naphthalene (1.0%), and their derivatives, as well as acenaphthene (2.0%), fluoranthene (3.1%), and their derivatives were identified. Heterocyclic compounds were represented by pyridine, acridine, and thiophene derivatives. Small amounts of long-chain n-alkanes were also observed.
Taking into account the composition, the heptane soluble and distillate fractions can be used as a feedstock for obtaining valuable chemicals, motor fuels, and carbon materials.
3.2.3.3. Benzo(a)pyrene Analysis
Toluene-soluble fractions isolated from the pitch-like products were analyzed via liquid chromatography to determine the BaP content as a marker of carcinogenicity. Its concentrations in the original solvents were from 0.59 to 8.1 mg/g, with CT solvent having the highest and HGO having the lowest concentration (Fig. 5).
The measured BaP concentrations in the pitch-like products ranged from 0.29 to 4.92 mg/g, which are much lower than those in the corresponding solvents. The BP concentrations in the pitch-like products calculated by additivity were significantly higher than the measured ones. This means that the BaP molecules contained in the solvent are partially chemically converted during the coal dissolution reaction, in contrast to high-temperature coal coking, which generates BaP. Increasing the coal dissolution duration from 1 hour to 3 hours (in the mixed solvent) resulted in a further decrease in BaP concentration from 1.84 mg/g to 1.48 mg/g. All pitch-like products obtained had lower BP concentrations compared to commercial pitches (up to 40 times compared to CTP).
CONCLUSION
The liquid-phase reaction of bituminous coal with commercially available heavy hydrocarbon fractions of coal- and petroleum origin at a moderate temperature of 380°C and autogenous pressure of 1.4-2.5 MPa resulted in deep and selective conversion of the organic coal matter into quinoline-soluble substances (to more than 80%), with only a small yield (0.4-0.6%) of gaseous products (CO2 and CH4 mainly).
The main dissolution products represented typical pitch-like matter with softening points of 80 to 90°C. Their properties, chemical composition, and molecular structural parameters depend on the type of coal and solvent used. The product obtained with coal tar as solvent was highly aromatic. Its aromatic nuclei consisted of predominantly polycondensed sparsely substituted cycles. The product obtained with petroleum-derived solvent was less aromatic, and the aromatic units were highly alkylated with fairly long alkyl chains. The pitch-like product with the intermediate structural parameters was obtained using a binary solvent.
The heptane soluble fractions and distillate fractions separated from the pitch-like products represented concentrates of the valuable polycondensed aromatics and heterocyclic compounds that can be used in the chemical industry.
All the pitch-like products had much lower concentrations of BaP, the smallest concentration showing the product obtained using petroleum-derived solvent (40 times less than typical coal-tar pitch). An increase in dissolution duration led to a further significant decrease in BaP concentration in the product.
In terms of composition, pitch-like products obtained through low-temperature dissolution of coals can serve as low-carcinogenic polyaromatic substitutes for coal tar. By selecting solvents and coals, it is possible to optimize the molecular-structural characteristics of the dissolved products in order to obtain chemicals and feedstock for the production of valuable carbon materials, including needle coke.
We anticipate the success of obtaining pitch-like products by coal dissolution under mild conditions to be an important key to the innovative development of the production of new low-carcinogenic polyaromatic feedstock for high-tech carbon materials.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: G.S.: Study conception and design; P.K., P.B.: Conceptualization; L.K.: Data curation; N.J., E.K.: Analysis, conceptualization, and interpretation of results; A.T.: Visualization; B.A., N.N.: Draft manuscript; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| FTIR | = Fourier Transform Infrared Spectroscopy |
| GC-MS | = Gas Chromatography-Mass Spectrometry |
| TG | = Thermogravimetric |
| CT | = Coal Tar |
| AO | = Anthracene Oil |
| HGO | = Heavy Gas Oil |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
FUNDING
This work was supported by the Russian Science Foundation under Grant (24-43-03001) and the Science and Technology Foundation of Mongolia (ShUTBIHHZG-2024/207).
ACKNOWLEDGEMENTS
The authors thank Dr. S. Novikova for recording FTIR spectra. The facilities of the Krasnoyarsk Regional Center of Research Equipment of Federal Research Center “Krasnoyarsk Science Center SB RAS” were used in this work.
DISCLOSURE
Part of this article has recently been published at https://www.mdpi.com/1996-1944/18/7/1660.


