All published articles of this journal are available on ScienceDirect.
Modern Approaches to the Enrichment and Engineering Processing of Poor Phosphate Ores
Abstract
The article analyzes a large number of published materials on mineralogical and geochemical data for global and Kazakhstan phosphorite reserves, as well as methods of their processing. Early studies were continued by studying the physicochemical features of phosphate ore deposits in terms of lithology and geochemistry. It has been established that Karatau rich phosphorites are the primary marine geosynclinal sediments, deposits of phosphate minerals from natural layers of seawater. The basis for the formation of the mineralogical structure was the movement of the Earth's crust, as well as certain conditions of the seabed relief and the adjacent land. According to information sources, the features of the mineralogical structure of new phosphorite deposits involved in the production of phosphorus and phosphorus-containing compounds are summarized. Comparative information data on the production of yellow phosphorus and its compounds at the only phosphorus plant in the Commonwealth of Independent States (CIS) are presented. Improvements in the wet and dry processing of phosphate ores have been analyzed to identify promising methods for producing phosphorus and mineral fertilizers. An analysis of existing methods for recycling phosphorus production waste, which provides a high level of environmental protection, was carried out. Considering the depletion of rich phosphorite deposits, an analysis of modern methods for enriching and processing poor phosphate ores was presented. Based on the analysis of poor phosphate ores and the physicochemical characteristics of phosphatized shales, methods for improving agglomeration using various hydrocarbon wastes as a fuel component were proposed. At the same time, considerable attention was paid to the methods of disposal and regeneration of solid and gaseous waste.
1. INTRODUCTION
Kazakhstan possesses some of the largest phosphorite reserves within the CIS, with total estimated resources of approximately 16 billion tons. A significant portion of these reserves is located in the Karatau Basin, where proven reserves amount to 2.5 billion tons. These deposits are notable for their high P2O5 content, ranging from 25% to 30%—the highest among CIS basins. The most important deposits in this region include Zhanatasskoye and Chulaktauskoye. Overall, Kazakhstan's estimated phosphorite resources exceed 15 billion tons. Numerous scientific studies describe phosphorite and apatite ores in 10–12 major deposits. In some areas, apatite occurs as part of complex ores, such as apatite-magnetite, rare-earth, and other mineral combinations. The P2O5 content varies significantly across different fields, from as low as 4–6% to over 20%, and in some cases reaching as high as 42% [1, 2].
High production capacities for the production of yellow phosphorus and mineral fertilizers in Kazakhstan emerged in the 1960s-1980s, when electrothermal and extraction processes were used to process the world's richest deposits of phosphorus ores in Karatau. Phosphorus in phosphate ores is combined with other elements in the form of phosphate minerals, including common groups of calcium phosphates and apatites [2, 3].
Ensuring a stable supply of phosphates for the production of phosphorus, its acids, salts, and mineral fertilizers, including the processing of substandard raw materials, is a pressing issue today.
The Karatau phosphorus ores are characterized by a length of more than 120 km and the presence of productive strata, the reserves of which amount to more than 1.2 billion tons. Geological studies of these areas have revealed that there is a powerful phosphorite stratum extending up to 70 meters along the northeastern branch of the Karatau. Moreover, the most interesting industrial accumulations of phosphorites turned out to be not in the area of Zhetymtal Mountain, where rocks with a high content of phosphoric anhydride were first discovered by I. Mashkara, but much further to the northwest, in the basin of the Koksu and Ushbas rivers [4-7].
Phosphorus ores are widely distributed in more than 65 countries of the world, but not all deposits have been developed on an industrial scale. According to recent research analyses, there are 6 categories of phosphorus-bearing provinces in the world: Asian, Australian, Arabian, African, East American Coastal Plain, and East European Platform. The most significant deposits containing more than half of the world's reserves of highest quality phosphorites are located in Asian, Arabian, and African regions, which are considered suitable for open–pit mining [8-13].
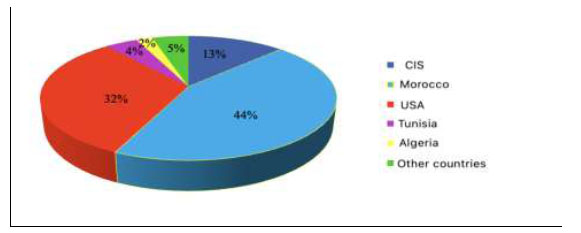
The largest producers of phosphorus and its compounds are the CIS countries, the USA, and Morocco, which account for 78% of the total global production. Phosphorite deposits are found practically all over the globe [14-17].
Considering the depletion of rich phosphorite deposits, large, poor ores of the Aktobe group of Chilisai phosphorites are currently widely involved in the processing in the Asian region [18].
The Aktobe phosphoriferous basin, which includes the deposits Chilisayskoye, Bogdanovskoye, Kandagachskoye, Novoukrainskoye, Pokrovskoye, Alginskoye, and Koktobinskoye, covers an area of more than 600 km2 with a content of up to 10% P2O5 [19, 20].
The article presents a comprehensive analysis of current trends in the enrichment and processing of phosphate ores, including studies of methods of acid decomposition, mechanochemical activation, flotation, heat treatment, and agglomeration. Special attention is paid to Kazakhstan's Karatau and Chilisai phosphorite deposits, which have industrial potential but require modernization of low-grade processing technologies.
This review necessitates the transition to integrated and low-waste technologies that ensure efficient use of resources and minimize environmental impacts. These developments are crucial for the development of the phosphorus industry in terms of attracting poor ores and rocks associated with the depletion of rich phosphorus reserves.
2. METHODOLOGY
The main international abstract and bibliographic databases (Scopus, Google Scholar, etc.), which are the leading information systems for the global scientific community, were used for the systematic collection of literature. The time range of the search was 1965-2024; publications in Russian and English were considered.
The criteria for inclusion and exclusion of publications were as follows: peer-reviewed articles and materials were included, with full access to the text in Russian or English. Dissertations, theses, monographs, and technical reports were excluded.
The selection process of publications was carried out in several stages. Initially, several hundred materials were found based on the formulated queries. Then duplicates and irrelevant publications were removed from this selection. At the second stage, screening was performed based on headings and annotations. After that, the number of documents was reduced to several hundred.
Classical methods of literature analysis were used to process and interpret the data collected. Descriptive and comparative analyses of the results were conducted, as well as thematic analysis to identify key research areas.
3. GENERAL CHARACTERISTICS OF THE WORLD'S PHOSPHATE
Studies have established that Karatau formation phosphorites are the primary marine geosynclinal sediments in the Asian group. The study of their composition and microstructure clearly showed that the deposition occurred through the direct chemical precipitation of phosphates from natural layers of seawater. The main prerequisite for the accumulation of Karatau phosphorites and other chemical sediments appears to have been the movement of the Earth's crust, which created certain conditions for the relief of the seabed and adjacent land. Under these conditions, the formation of hydrated phosphate compounds is possible. After its deposition at the bottom of the basin, phosphate sediments underwent complex secondary changes. The rocks of the phosphorite-bearing stratum have undergone special changes in places under the influence of granite intrusions. Phosphorites were subjected to thermal metamorphism and transformed into stratified apatites [21-28].
The main reserves are concentrated in the Zhanatas, Aksai, Koksu, Kokjon, Akzhar, Uchbas, Geres, Tiesai, and Chulaktau deposits, which contain the following types of ores in the Karatau basin.
- Rich phosphorite ores containing more than 28.7% P2O5 are used for grinding unenriched phosphorite flour. Rich ores were located in the surface zones of the Zhanatas (south-east), Kokjon, Koksu, Geres, and Aksai deposits, consisting of phosphate grains, oolites, and phosphate-cemented fragments.
- Ordinary phosphorite ores containing 23.0–28.7% P2O5, from which phosphorus can be obtained without enrichment, were located in the deposits of Chulaktau, Zhanatas, Tiesai, and Koksu. In the Kokjon and Aksai deposits, they are predominantly carbonate, while the remaining deposits contain more silica and fewer carbonates with a characteristic subtle interpenetration of phosphate with siliceous minerals.
- Poor phosphorite ores containing 15-23% P2O5 are located in the Akzhar, Uchbas, Chulaktau, and Koksu deposits [3, 12, 19].
According to previous studies, deposits consisting of granular phosphorite ores account for more than 50% of the world's phosphate raw material reserves [29-31]. The Arabian-African deposits, which are considered the richest area, contain most of the reserves of granular phosphorites with a volume of approximately 5100 million tons. P2O5 includes countries such as Algeria, Egypt, Morocco, Tunisia, and other countries. All deposits belong to the Northwest African basin. This is the largest Khuribga deposit in the world. The estimated reserves exceed 32 billion tons; the proven reserves amount to 16.3 billion tons. The mass fraction of P2O5 phosphorus is on average 29.3%, the thickness of the formations is from 1 to 2.5 m, and the total thickness of the phosphorite formation is 40-60 m. According to the analysis, the mass fraction of P2O5 averages 27.4%. The vast majority of reserves can only be developed underground. In addition to onshore deposits, large offshore phosphorite deposits have been discovered in Morocco, located offshore in the southern part of the country's water area, where the mass fraction of P2O5 in them is 19%.
Florida and Georgia basins are the most important in the United States in terms of production and reserves of phosphorous ores. This is the oldest phosphorous-bearing basin. The first deposits were discovered here in the 1880s. The vast majority of reserves are located in the fields of Florida, with the most significant being North Florida, Central Florida, and North-Central Florida. The heavily used large deposits of South and East Florida have been developed for more than 100 years [28].
It follows from the work of N.G. Peshev [32], that the Asian continent has significant resources of phosphorites. The largest reserves are in China; they are also substantial in Mongolia, Jordan, and Iraq. The total reserves of phosphorites in China in the late 1990s were estimated at more than 9 billion tons, of which 1.2 billion tons are proven reserves. Approximately 4% of the reserves are phosphorites with a mass fraction of P2O5 of more than 30%. The rest are of medium-quality phosphorites (15-30% P2O5). A huge part of the reserves is contained within the South China Basin (Yunnan, Guizhou, Hunan provinces), where phosphorites lie in thick layers (3-7 m).
The Palabora deposit was discovered in the northern part of South Africa. According to forecasts, its reserves exceed 2.4 billion tons with a mass fraction of P2O5 of 7-11.5%, and estimated reserves are estimated at 10 billion tons. Some sites contain ores with a high mass fraction of P2O5 (34-40%) [33, 34].
Table 1 summarizes the main characteristics of important igneous phosphate deposits worldwide, showing that the P2O5 content varies and ranges from 3 to 38%. Unlike sedimentary phosphorites, igneous phosphate ores are more economically important because they offer high-quality phosphates with minimal concentrations of undesirable pollutants (Cd, As, Pb, Si, and Al). They may be related to the economic concentrations of certain strategic elements, including rare earths, niobium, copper, titanium, zirconium, uranium, vermiculite, and iron Table 1 [35, 36].
This table shows the diversity of P2O5 content in phosphate ores from various locations, including Kazakhstan, and uses enrichment levels to reduce phosphate content in the ore.
A characteristic feature of Kazakhstan's low-grade phosphorites is the presence of large amounts of iron and aluminum-containing minerals, as well as other related impurities, including carbonates and organic compounds in the form of phosphate-siliceous shales. The main methods of processing low-grade phosphate raw materials are: (i) partial replacement of high-quality phosphate raw materials with low-grade ones in the production of extraction phosphoric acid, superphosphates, and nitric acid extraction, (ii) decomposition of phosphate raw materials with a reduced rate of mineral acids, (iii) preparation of phosphorous flour based on phosphorites of the jelly type, and (iv) “dry” or “wet” mechanochemical activation of phosphorites, including in a mixture with mineral and (or) organic additives [6, 7, 37, 38].
In the CIS, the main producers of phosphorus-containing and feed phosphorites are Kazakhstan, Russia, Uzbekistan, Ukraine, and the Baltic States (13%). The resources of phosphorous raw materials in the CIS countries are represented by exploratory forecast reserves. In terms of their number, the CIS ranks third in the world, second only to Morocco (44%) and the USA (32%). The proven reserves amount to about 2 billion tons of P2O5. They are concentrated in Kazakhstan (35.3%), Northwestern (35.2%), Volga-Vyatka (14.7%), Central (3.8%), East Siberian (6%), and other economic regions of Russia, as well as they are also present in Central Chernozem (0.6%), Volga (0.1%), Ural (0.3%), West Siberian (1.1%) regions of Russia, and Estonia (3%). The estimated reserves in the CIS are about 8.1 billion tons of P2O5 (Fig. 1) [28, 39].
| Country and deposit | Content of P2O5 (%) | Main characteristics |
|---|---|---|
| Russia (Khibiny) | 15 | High nepheline content, large phosphate deposit |
| Russia (Kovdor) | 6.5 | It is associated with magnetite, a deposit with a low concentration |
| South Africa (Palabora) | 8 | It is rich in vermiculite and chalcopyrite, and is widely used for fertilizers. |
| Brazil (Jacupiranga) | 5 | Calcite-dominated carbonatite |
| Finland (Siilinjarvi) | 3.5 | Low content associated with calcite and phlogopite |
| Zimbabwe (Dorowa) | 6 | Carbonatite with low phosphate content |
| Sri Lanka (Eppawala) | 38 | An exceptionally highly graded deposit |
| Canada (Cargill) | 20 | Moderate content, enriched with pyrochlore |
| Kazakhstan (Karatau) | 15-25 | Large deposit of phosphorites, significant reserves |
| Kazakhstan (Chilisai) | 9 | Deposits with moderate phosphate content |
| Kazakhstan (Enriched phosphorites) | 20-30 | After enrichment, the P2O5 content is expected to reach 20-30% |
| Kazakhstan (Landfill waste from Zhanatassky factory) |
15 | Enrichment waste with a P2O5 content of about 15 |
| Kazakhstan (Landfill waste from the Karatau factory) |
14-17 | Enrichment waste with a P2O5 content of 14-17% in particular |
The potential global reserves of phosphorite and apatite ores are shown in Fig. (1).
In recent years, both well-known types of phosphorite and apatite deposits, well-established in the CIS countries and new foreign types, differing in the content of the main component, have been involved in the production of phosphorus and its compounds, as well as various mineral fertilizers (Fig. 2) [6].
1-Khibiny apatite concentrate of JSC Apatite, 2- Apatite concentrate of Kovdorsky Mining and Processing Plant (MPP), 3-Phosphorite concentrate of Kingisepp JSC Phosphorite, 4- phosphorite concentrate of JSC Phosphates Moscow region, 5- phosphorite concentrate of JSC Karatau, 6-phosphorite concentrate Chilisai, 7-Jordan phosphorite concentrate El Hasa deposits, 8- Algerian Jebel Onk phosphorous concentrate, 9-Tunisian Metlaoui phosphorous concentrate, 10- Vietnamese apatite concentrate from Laokai deposit, 11-Mongolian Khubsugul phosphorous concentrate, 12- Mongolian phosphorus concentrate from the Burenkhan deposit, 13- Moroccan phosphorus concentrate from the Khuribga deposit, 14- Syrian phosphorus concentrate from the Kneifis deposit
Analysis of the obtained diagram showed that in the CIS countries, rich phosphorous concentrates are characteristic of the Khibinsky, Kovdorsky, and Kingisepp deposits. Jordanian, Vietnamese, Mongolian, and Moroccan phosphorous concentrates are slightly inferior in terms of the content of the main component P2O5. The CaO content in all the studied deposits ranges from 44 to 52%. Phosphatide concentrates from the Karatau and Chilisai deposits are lower in both the content of the main components, P2O5 and CaO, than in the CIS countries and new deposits in the African and Asian regions [6].
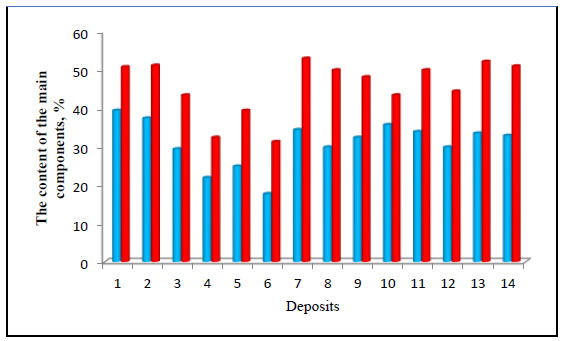
Diagram of the composition of phosphate raw materials in terms of the content of the main components P2O5 and CaO [6].
4. PROMISING TECHNOLOGIES FOR THE ENRICHMENT AND PROCESSING OF PHOSPHATE ORES
In nature, phosphate minerals are first formed in the crystal lattice of which carbon and hydroxyl groups are concentrated. These minerals have a high content of the phosphate part; they dissolve in soil acids (the so-called citric-soluble phosphate). Ores containing such minerals are used as fertilizers in the form of phosphorous flour.
Phosphate minerals are similar in composition to fluorapatite, Ca10(PO4)6F2. Phosphate minerals differ from apatite in that part of the phosphorus is isomorphically replaced with carbon or sulfur, and sometimes hydroxyl groups also enter the crystal lattice [40].
The main minerals of phosphorites are francolite (Ca(Н2O)10(F, OH)2(PO4,CO3)6 and carbonatapatite (Ca10P5 CO23(OH)3).
If large quartz is present in phosphorites, they are enriched by selective grinding. Soft phosphate is easily crushed into a fine-grained product, while quartz concentrates into a coarse-grained product. The phosphate part is separated from quartz by screening [41].
Wet enrichment processes involve a combination and alternation of preparatory operations (ore repulpation, selective disintegration, and surface scrubbing of phosphate grains) with separation operations (grading on screens and hydraulic desalination), followed by flotation and firing.
The flotation process used for the enrichment of carbonate magnesium-containing ores (Kazakhstan, Russia) has become the most widespread; however, the proximity of the flotation properties of phosphate and host rocks, fine inclusions, and low efficiency of the flotation reagents used do not allow achieving high concentrate quality and loss reduction [42-47].
Kalauova and Sadyrova [48], have developed a fundamentally new technology for processing low-grade phosphate ores, which enables the extraction of phosphorus (V) oxide up to 97%, comprehensively uses waste magnesium, gypsum, and quartz in various industries, and the obtaining of highly effective phosphorus concentrate and long-acting magnesium phosphorus fertilizer. The above technological scheme of complex processing of dolomitized phosphate raw materials is resource-saving and allows for improving the quality of the concentrate due to the stage of chemical enrichment, improving extraction rates during the processing of concentrates of chemical and Chemical Flotation Enrichment (CFE), to obtain target products from liquid and solid enrichment waste. A characteristic feature of the proposed scheme of chemical flotation enrichment of Karatau ores is the use of waste as a source of phosphate ions in the production of Monoammonium Phosphates (MAP) [49].
A wide variety of technological types of phosphorite ores has led to the proliferation of combined enrichment schemes. Promising methods for processing and enriching phosphorites include separation in heavy suspensions, sorting, heat treatment, magnetic separation, and bacterial leaching. The initial stage involves auxiliary operations, such as crushing, screening, grinding, desalination, filtration, and drying. The extraction of phosphate minerals from the tailings of the main washing process made it possible to increase the total extraction of P2O5, thereby contributing to an increase in the complexity of the use of phosphate raw materials [50, 51].
The main issue of phosphorous ore flotation is the reagent regime: type, consumption, and combination of reagents used. As collectors, the authors use various surfactants with a solitophilic polar group capable of interacting with the surface of certain mineral particles, as well as reagents with a carboxylic polar group [52, 53]. Based on the conducted preliminary flotation studies, the following conditions were selected: the fineness of the grinding was 42.5% of the “- 0.063 mm” class; soda (1.5 kg/t) was used to create the pH of the medium, which was fed into the flotation head; liquid glass (1.5 kg/t) was fed into the flotation head along with soda to depress the waste rock. Cheap and affordable mixtures of tallow oil and kerosene are used as anion-active collecting reagents for the flotation of phosphate-containing ores. At the same time, a concentrate of the II main flotation was obtained with a P2O5 content of 24.2%, which can be a raw material for the production of phosphorous fertilizers. The content of P2O5 in the tailings is 0.10% [54].
In contrast to the generally accepted methods of phosphate enrichment, where hydrocyclone classification is primarily used to remove ultrafine particles in the form of tailings, the method proposed by Teague and Lollback [55] made it possible to extract a high proportion of ultrafine particles by flotation without prior separation.
A number of variables and their effects on the flotation extraction of ultrafine phosphate have been studied, including pulp density and water quality during conditioning and flotation, the type of flotation machine, and reagents used to reduce the content of Fe2O3 and Al2O3. Some excellent results were achieved using samples containing up to 75 weight.% of particles with a size of 20 microns, with the extraction of up to 91.2% P2O5 to a concentration of 34.7% P2O5 from raw materials with a low content of 6.46% P2O5 and the extraction of 92.4% P2O5 to a concentration of 30.2% P2O5 from raw materials with a content of 10.6% P2O5. The proposed method is designed to extract phosphate from ores containing particles up to 80 by weight.% larger than 20 microns, by flotation using a Jameson cell.
It is known that in order to achieve high technological and economic performance of the calcium phosphate reduction process, phosphorite, quartzite and coke must be homogeneous in granulometric composition, free of free and bound water, contain a minimum amount of carbonates and various harmful impurities (Fe2O3, Al2O3, K2O, Na2O, sulfur of organic substances), and phosphate raw materials have high the content of P2O5. Phosphorous ores extracted for the production of phosphorus, as a rule, contain moisture, hydrates, carbonates, and various impurities. Therefore, the raw materials must be subjected to special treatment before being fed into the furnace in order to ensure they meet the quality requirements of the technology. Depending on the quality of the raw materials, the preparation process includes drying, decarbonization, and consolidation of small fractions of the raw materials, among other steps. However, when preparing raw materials, it is practically impossible to change the content of such oxides as P2O5, CaO, SiO2, Al2O3, Fe2O3, Na2O, and K2O in it, therefore, a given amount of P2O5 and the maximum allowable amount of these components must be provided during the extraction and enrichment of raw materials [56, 57].
It was in the 60s that the chemical industry of Kazakhstan flourished. An analysis of the data showed that in 1950, when production of mineral fertilizers began in the republic, approximately 22 thousand tons were produced, and by 1977, this had increased to more than six million tons. The intensive development of the Karatau phosphorite deposit played a huge role in this.
In the following years, the chemical, lithological, and mineralogical compositions of the initial phosphate ores were studied to determine the values of the mechanical and thermal properties for electroplating. The data was recorded in the mining plans and transmitted to the phosphorus production plant laboratories to determine the effect on the operation of phosphorus furnaces and the quality of the phosphorus obtained. Similar work was carried out on phosphate raw materials for sulfuric acid production. As a result, new additional criteria for assessing the suitability of ores for basic processing methods were created. In these areas, close creative relations have developed with the phosphorus plants of Dzhambul, Shymkent, and Tolyatti, as well as the chemical plants of the Soyuzphosphor association, including those in Almalyk and Samarkand [1].
According to scientists, the specific composition of ordinary and low-grade Karatau phosphorites involved in the production sector requires fundamentally new technical solutions. In this regard, the researchers noted two possible approaches: the first is to bring the composition of phosphorites up to the requirements of traditional technologies, the second involves the creation of such methods in which the composition and its fluctuations do not significantly affect technological performance [38].
In recent years, in Kazakhstan, as in Russia, fertilizer production has become an export-oriented business with a significant difference in scale [58-60]. The applications of phosphates are numerous, but more than 80% of the phosphates extracted worldwide are used for the production of phosphorous fertilizers. With an increase in the Earth's population and an increase in food demand, the need for the production of phosphorous fertilizers increases by 2-3% annually [61].
It has been established that it is the demand for phosphorous and complex fertilizers that determines the dynamics of the phosphate market. The Karatau basin is the only ore base in the Asian region of the CIS that supports further industrial processing at phosphorous and other chemical enterprises in Kazakhstan, Uzbekistan, Turkmenistan, and Russia [40, 61-65].
In the current conditions, given the high energy intensity of the yellow phosphorus production process and the presence of a large amount of low-grade raw materials and waste output, these factors are a significant barrier to the development of the industry. Almost three-quarters of the total production of phosphorus and its compounds is concentrated in China, which has substantial reserves of phosphorous raw materials and cheap electricity [66].
The production of phosphorous chemicals begins with yellow phosphorus, so access to resources is of great importance, and the price of yellow phosphorus has a direct impact on the price of derivative products. The cost of electricity accounts for more than 70% of total production costs. Yellow phosphorus production is a very energy–intensive process (producing a ton of yellow phosphorus requires an average of about 14500 kWh), and China has an advantage in producing energy-intensive products as a result of low electricity prices ($0.25 per kWh). Leveraging this advantage, China has expanded the production of yellow phosphorus and is successfully conquering export markets. China exports large quantities of yellow phosphorus to countries such as Japan, India, and the United States [67].
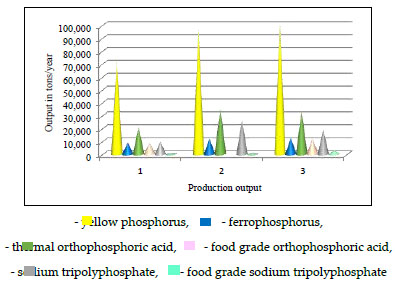
In Kazakhstan, high rates of phosphorus production and sales were achieved due to the high quality of marketable products, competitive prices, and organized supply services, as well as the only Novodzhambul Phosphorous Plant (NDРР) in the CIS, a branch of Kazphosphate LLP. NDРР is a chemical plant for the production of yellow phosphorus and phosphorus-containing products. The company operates the following main production facilities: phosphorous agglomerate, yellow phosphorus, thermal orthophosphoric acid, sodium tripolyphosphate, sodium hexametaphosphate, granular thermophosphoric slag, ferrophosphorus, food-grade orthophosphoric acid, and food-grade sodium tripolyphosphate [1, 2].
The technological process for the production of yellow phosphorus and its compounds begins with the preparation of an agglomeration charge (phosphorite containing more than 22.0% P2O5, coke and its substitute containing at least 80% C, and quartz–containing material), sintered on sintering machines to produce an agglomerate cake [68, 69].
The yellow phosphorus production process involves reducing the charge melting in ore-thermal furnaces to produce a gaseous state, purifying phosphorus-containing furnace gas in electrostatic precipitators, and condensing phosphorus from the purified phosphorus-containing furnace gas in scrubber-type condensers irrigated with water inside. Discharge of slag and ferrophosphorus from ore-thermal furnaces with slag granulation by utilization of phosphorus-containing dust in fertilizer [70]. Yellow phosphorus—after settling and separating from sludge with subsequent purification from organic impurities—is sent to the production of Thermal Phosphoric Acid (TPA), Food Grade (FG), as well as sodium polyphosphate and calcium phosphate (Fig. 3).
In the period from 2017 to 2019, the company achieved the status of the most promising plants in the CIS for the production of yellow phosphorus and its compounds.
Comparative information on the production of the main product of yellow phosphorus and its compounds at the NDPP in the period 2017-2019 showed that the output of the main product, thermal phosphoric acid, food grade orthophosphoric acid, and food grade sodium tripolyphosphate is steadily increasing. These facts reflect the high demand for the plant's products for the main types of products in both domestic and foreign markets [2, 37]. Fig. (4) shows the data on the actual output for 2017-2019.
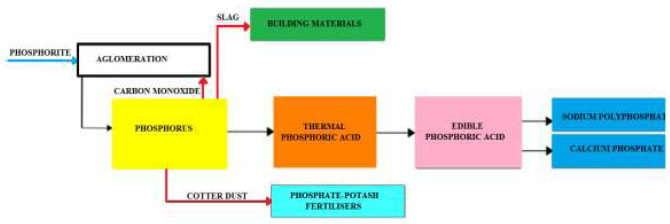
Schematic diagram of phosphorus production and its compounds [70].
The branch of Kazphosphate LLP, the Mineral Fertilizers Plant in Taraz, is a chemical plant that produces sulfuric acid, extracts phosphoric acid, ammophos, sulfoammophos, tricalciphosphate, feed-grade defluorinated phosphates, and phosphogypsum. In 2022, the launch of the second Ammophos-2 fertilizer production line was announced, enabling an increase in the production of finished products to 1 million tons per year.
In recent years, the production of environmentally friendly ammonium dicalcium phosphate, long-acting ammoniated calcium phosphate, feed tricalcium phosphate, organomineral fertilizer sulfocarbon based on sulfocarbon dust and nitroammophos has been mastered. The manufactured products are sold on the domestic market and in the markets of Europe, the CIS, and Central Asia [37, 71, 72].
In order to establish the basic physico-chemical patterns and develop processes for the acid processing of low-grade phosphorites and man-made waste in the CIS, an analysis of the current state of the phosphate raw material base in different countries and the mineral fertilizer market was carried out [73].
Phosphate raw materials have been studied: phosphorites from Polpinskoye (PP), Kyzylkumskoye (KyzP) deposits, Karatau phosphorites from the Koksu (KSP) and Kokjon (KJP) deposits, apatite concentrate from the Khibinsky deposit (KHAK), which is widely used in the domestic segment of mineral fertilizer production, as well as waste from the phosphorous industry of the Republic of Kazakhstan – Phosphorous Sludge (PS) and Cottrell Milk (CM), the chemical composition of which is given in Table 2.
| Content, % weight | P2O5 | CaO | MgO | Fe2O3 | Al2O3 | CO2 | Cl | F | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| PP | 15.30 | 27.42 | 0.23 | 2.07 | 0.90 | 5.00 | - | 0.010 | 28.4 |
| KyzP | 17.27 | 45.52 | 1.26 | 0.56 | 1.06 | 15.5 | 0.178 | 0.087 | 2.12 |
| KSP | 21.39 | 35.72 | 0.97 | 0.91 | 0.95 | 3.95 | - | 2.79 | 28.27 |
| KJP | 26.13 | 45.28 | 1.84 | 0.68 | 0.97 | 8.78 | - | 1.93 | 8.43 |
| KHAK | 39.10 | 50.35 | 0.60 | 0.44 | 1.05 | - | - | 3.20 | 1.50 |
| PS | 17.01 | 13.74 | 4.16 | 0.81 | 1.08 | 15.2 | - | 0.47 | 45.38 |
| CM | 23.05 | 8.99 | 1.29 | - | 2.66 | - | - | - | 25.08 |
All the presented samples in terms of the content of the target component belong to poor, difficult-to-enrich phosphate raw materials with admixtures of carbonates, iron, aluminum compounds, and acid-insoluble residue. The presence of carbonates in the phosphate substance’s composition of the ore suggests its higher reactivity to acids compared to apatites, and at the same time, the predictable problem of foaming.
The high content of impurities (calcium and magnesium carbonates, iron, aluminum compounds, clay, and silicate minerals) in comparison with the content of the target component in phosphate ores of sedimentary origin makes their chemical processing for mineral fertilizers unattractive. Therefore, mechanical operations are often employed, such as crushing and grinding of phosphorite, resulting in a polydisperse product used as a low-grade fertilizer with prolonged action. This work is devoted to the search for a solution to this problem and is aimed at substantiating the possibility of obtaining phosphorus–containing fertilizers from low-grade phosphorites (15-26% P2O5) of the Polpinskoye, Karatausskoye, and Kyzylkum deposits, as well as man–made waste from the phosphorus industry (17-23% P2O5) using industrial schemes and technologies for processing domestic apatite concentrate by direct acid extraction [74, 75].
Kydyralieva et al. [76], showed the possibility of efficient processing of phosphorus-containing technogenic waste from the Republic of Kazakhstan, including phosphorous sludge from Kainar LLP and Cottrel milk from Kazphosphate LLP. Optimal conditions for the technological process have been determined, and schematic diagrams for the production of fertilizers, such as superphosphate (more than 39%), ammonium phosphate (34%), and associated low-cost, low-concentration NPK fertilizer (14%) for local use have been proposed.
The results of research and industrial implementation of a method for modifying ammonium nitrate with siliceous additives have shown an increase in the strength of the granules, as well as an increase in their phase and thermal stability. The results of studying the chemical composition and properties of technogenic waste from the phosphorous industry of Kazphosphate LLP and Kainar LLP made it possible to establish the technological basis for their processing into phosphorus-containing fertilizers [77].
Using nitric acid technology, a sample of complex NPK fertilizer with a total nutrient content of about 28% was obtained; four types of balanced complex fertilizer were obtained by combined nitric acid decomposition, one of which is chlorine-free NPK fertilizer, which is in demand in animal husbandry and greenhouses (due to the irremovability of soils). Three types of complex NPK-enriched fertilizers containing over 41% of nutrients were obtained by the nitric-phosphoric acid technology of PP processing, with the release of insoluble residue from reaction systems upon completion of the decomposition process [78].
5. PHOSPHORUS INDUSTRY WASTE RECYCLING
The objective of the Environmental Code of the Republic of Kazakhstan is to ensure a high level of environmental protection through the implementation of state regulation aimed at preventing environmental pollution, preventing environmental pollution in any form and ensuring the elimination of the consequences of damage caused, as well as the gradual reduction of negative anthropogenic impact on the environment [79, 80].
A research study by Litvinova and Suchkov [81], is devoted to solving the pressing issue of the beneficial utilization of multi-tonnage man-made waste from the mineral resource complex. The waste of processing phosphate raw materials, phosphogypsum, whose main composition is CaSO4·2H2O, was chosen as the object of research. The paper analyzes the material composition of phosphogypsum from various origins, including fresh and landfilled sources. It has been confirmed that the composition of phosphogypsum, due to its man-made origin, has several differences compared with gypsum-containing raw materials of natural origin. The waste contains products of the incomplete decomposition of phosphate raw materials, heavy metal oxides, and compounds containing valuable components, such as rare earth elements. From an ecological perspective, phosphogypsum is a source of complex negative effects on the atmospheric air, soil, underground, and surface water bodies. An analysis of the methods of proper utilization of phosphogypsum has shown the effectiveness of its complex processing, which involves the extraction of valuable components via the carbonate conversion method. The method indicated by the authors in the conditions of the production cycle can also be complemented by the utilization of industrial flue gases containing CO2, which helps reduce the carbon footprint. The introduction of such solutions contributes to a more rational use of the country's mineral resource base and to an increase in the efficiency of its reproduction, as well as to a reduction in environmental pollution.
Idrissi et al. [82], investigated the characteristics of nine different phosphate mine wastes and their potential applications. These materials are usually extracted and disposed of in landfills. Their research has shown the potential use of these materials based on their chemical and mineralogical composition, as well as their thermal properties. Based on the results obtained, it was established that these wastes have great potential for various industrial applications, including traditional ceramics, the cement industry, geopolymers, alkali-activated materials, and acid drainage control in mines. Their high content of carbonates can be used to produce lightweight ceramic products with good acoustic and thermal insulation properties. In addition, due to their high content of CaO, SiO2, and Al2O3, some samples may be mixed with certain additives for use as clinker raw materials or additional binders. Furthermore, the high content of carbonates provides a high neutralization potential to stabilize the acid-forming tailings. The cost of such raw materials will reduce the consumption of natural resources in the construction sector, as well as solve environmental problems associated with these materials.
In China, as a result of preparing phosphate ores for processing, a large number of phosphate tailings are formed. These wastes are not fully disposed of properly and cause serious environmental and social problems. Previous studies suggest using phosphate mine tailings for the production of expanded clay materials that can be used as a building material. For processing, these wastes were mixed with a soft layer and materials from black slate [83-85]. Using single-factor experimental tests, the influence of the mass ratio of phosphate ore tailings, the content of soft intermediate layer and black shale, as well as firing parameters, such as preheating temperature, preheating time, sintering temperature, and sintering duration, on the properties of ceramisite was investigated. A one-factor experimental study revealed that the phosphorus content, water absorption rate, and particle strength exhibit different correlations under the influence of the material ratio and the firing process. The optimization test showed that the values of various factors for the properties of ceramisite are determined in the following order: sintering temperature > preheating temperature > sintering time > preheating time. Final technical parameters of ceramisite reaction surface determination methodology: preheating temperature 350°C, preheating time 9.6 minutes, sintering temperature 943°C, sintering time 60 minutes. When optimizing the process, the available phosphorus content was 9.17%.
Analysis of statistical data has shown that by the end of the first decade of the 21st century, the annual consumption of phosphate raw materials reached 166 million tons. This is due to the aggravation of the open phosphorus cycle. Despite the fact that the world's phosphate ore reserves are huge, they are among the depleted resources, and the natural return of phosphorus to the natural cycle does not compensate for its consumption.
Kaipova et al. [86], demonstrated the possibility of sulfate decomposition of substandard Karatau phosphorites. As a result of the research, complex fertilizers were obtained with a sulfuric acid concentration of 87% of the stoichiometry. Instead of sulfuric acid, ammonium sulfate was added in an amount of 10-20%. The amount of nutrients in fertilizers ranges from 34.35 – 67.82%.
An analysis of the information data showed that Ukrainian phosphate raw materials have a low phosphorus content of about 5-7%. However, they can still be used to produce phosphorus flour (the cheapest fertilizer), which is as effective as superphosphate on acidic soils (making them > 50% of the country’s land). The proven reserves of phosphate raw materials in Ukraine, if fully utilized, can theoretically provide ~ 45% of the utilization of existing capacity for the production of phosphorous products. Phosphorus-containing flour mixtures produced by Ukrainian plants are exported to Hungary, Poland, Slovakia, Israel, France, and Nigeria. In order to obtain a phosphorite concentrate, conditioned by the content of harmful impurities and suitable for sulfuric acid processing, Mozheyko et al. [87] suggest that the phosphorite ore should initially be condensed in hydrocyclones and hydraulic separators. After filtration and drying in drum dryers, P2O5 up to 77.6% was isolated from the condensed product.
Currently, due to the depletion of apatite ores, various phosphorous deposits are being utilized in the production of phosphorous fertilizers, which are quite suitable for production, but are currently not being practically involved. Meanwhile, the need to replenish the balance of nutrients in the soil indicates the need to incorporate phosphorite ores into the production of mineral fertilizers. Phosphorite ores, which have a significant variety of compositions, are inferior in many respects to apatite ores: the relatively low content of a useful component in the raw material and a large amount of impurities make both enrichment and chemical processing difficult. In this direction, scientists have studied various methods of enriching phosphorous ores. The results of another study made it possible to establish the inefficiency of the enrichment of various phosphorous ores through chemical leaching of impurities with weak organic acids [88]. In this case, one and a half oxides are extracted more slowly, and an increase in extraction time negatively affects the final product. The causes of foaming and the ways of stepwise decomposition are revealed. Researchers studied methods for optimizing the decomposition of nitric acid in low-grade phosphorites.
Gavrilyuk et al. [89] have shown the possibility of processing various grades of Moroccan phosphorites into extraction phosphoric acid and complex fertilizers through sulfuric acid decomposition.
Based on the results of chemical, microscopic and X-ray phase analyses of precipitation resulting from the acid decomposition of the proposed types of phosphate raw materials, it was found that the optimal conditions for the sulfuric acid decomposition process for phosphorites are: the concentration of sulfuric acid in the liquid phase is less than 25 weight %, the consumption rate of sulfuric acid is 100-105%, the temperature is 80°C, the duration is at least 2 hours. It has been established that differences in the quantitative composition of individual phosphorus-containing phases, particularly apatite and fluorapatite carbonate, have a significant effect on the nature, kinetics, and quantitative parameters of the decomposition process. Moroccan phosphorite of the K-9 brand is considered the most acceptable for processing.
Phosphorites of central Kyzylkums are processed at plants in Uzbekistan (JSC Ammophos-Maxam, Samarq and kime, and Kokand Superphosphate Plant) that produce phosphorus-containing fertilizers. Their phosphate raw materials are provided by the Navoi phosphorite complex, which produces washed burnt phosphoconcentrate (26% P2O5, 1.5% CO2, and CaO: P2O5 = 2.0) by thermal enrichment of Kyzylkum phosphorite ore (16.2% P2O5, 17.7% CO2, and CaO: P2O5 =2.85). Due to the decline in reserves of richer phosphorites, scientists worldwide are focusing on the processing of poor phosphorites into qualified phosphorus-containing fertilizers [90, 91].
Various activation methods are being developed, i.e., methods for converting the indigestible form of P2O5 in phosphate raw materials into plant-digestible forms. In this regard, the methods of mechanical, thermal, chemical, mechanochemical, and microbiological activation are promising, which make it possible to convert the indigestible form of P2O5 in raw materials into a form digestible for plants at the lowest cost [92, 93]. The authors propose using chemical and mechanochemical processing methods, which can convert poor phosphate raw materials unsuitable for acid processing into an effective phosphorous fertilizer.
Kurbanov et al. in their study tested the suitability of potassium chloride for the activation of ordinary phosphorous of Central Kyzylkum with a content of 18.7% P2O5 [91]. A significant effect was achieved by crushing the flour mixture with a ratio of P2O5: K2O = 1:0.7. An increase in the proportion of KCl in the flour mixture to a ratio of P2O5: K2O = 1:2 increases the relative content of P2O5 in citric acid to 47.32%.
In another study, to obtain activated NPK fertilizers with different ratios of nutrient components, activation was performed in the presence of granular ammonium nitrate (34% N), technical crystalline ammonium sulfate (21% N), and potassium chloride (60% K2O) [91]. Mechanochemical activation of phosphate raw materials in the presence of mixtures of nitrogen salts with potassium chloride at a ratio of N:P2O5:K2O = 1:0.5:0.3 showed an increase in the relative content of the digestible form of P2O5 to 75.97% using (NH4)2 SO4 and to 63.79% using NH4NO3.
A sharp reduction in apatite ore reserves at exploited deposits is accompanied by an increase in prices for high-quality phosphate raw materials. Under these conditions, it is advisable to involve low-grade phosphates in the processing process, which are characterized by a low content of the target component (P2O5) and a high content of impurities, such as carbonates, clay minerals, and insoluble residue. Acid processing of low-grade phosphate raw materials requires further research on decomposing components and a consumable agent for pre-enrichment of raw materials with acid processing [94].
At the current stage of development of the phosphorous industry in Kazakhstan, one of the urgent tasks is to develop the physico-chemical and technological foundations of low-waste energy-saving technology for the preparation of raw materials and waste processing of various enterprises that ensure resource conservation and product quality [95].
One of the problems of the phosphorus industry is the complex use of phosphate-siliceous raw materials from the Karatau basin. The involvement of off-balance phosphorites, phosphate-siliceous rocks, and phosphatized flints in industrial processing will expand the raw material base and reduce harmful effects on the environment [96].
Phosphate rocks are a vital resource for the global food supply and security. They are the primary raw materials for phosphoric acid and fertilizers used in agriculture, and are increasingly being considered as a potential source of rare earths. Phosphate rocks occur either as sedimentary deposits or as igneous ores associated with alkaline rocks. In both cases, the genesis of high-quality phosphate rocks is the result of complex concentration mechanisms involving several (bio)geochemical processes. Some of these ore formation processes remain poorly understood and are the subject of scientific debate. Morocco has the world's largest deposits of sedimentary phosphate rocks, and also possesses several alkaline complexes with the potential to contain igneous phosphate ores. This article summarizes the main geological features and driving processes of sedimentary and magmatic phosphates, as well as discusses their global reserves/resources situation. It also provides a comprehensive overview of published data and information on Moroccan sedimentary and igneous phosphates. He reveals significant gaps in knowledge and the lack of data, in particular regarding phosphate geochemistry and correlations at the basin scale [9].
Phosphate rocks are by far the most important phosphorus-containing raw materials used in the fertilizer industry. They are the primary source of Phosphorus (P), which is an essential element for agriculture and various industrial applications (for example, animal feed, cosmetics, and electronics) [34, 97]. Phosphate rocks are also likely to contain significant amounts of Rare Earth Elements (REE), which makes them a potential resource for REE, given the volume of their production worldwide [98, 99]. Phosphorus in phosphate rocks is always combined with other elements in the form of phosphate minerals, of which the most common and widespread belong to the apatite group [100]. Ensuring a stable phosphate supply is one of the most difficult challenges that requires proactive strategies, including the recycling of phosphate mining and processing waste, in addition to the exploration of new potential phosphate ore resources [101, 102]. Sedimentary phosphorites of marine origin are currently the main raw materials for the phosphate industry and account for a significant share of global phosphate rock production (≃90%) [103]. Igneous phosphate rocks account for ~10%, and the rest comes from residual and guano-type sedimentary deposits [34, 104]. Both sources (igneous and sedimentary rocks) have some advantages and disadvantages in terms of their chemical quality, geographical distribution, and operational suitability [34].
In addition to their economic value, phosphate rocks have high scientific value. Sedimentary phosphates provide valuable information about the ecology and chemistry of the world's past oceans [34, 105]. Indeed, their genesis, accumulation, and preservation require special paleoecological conditions and involve complex biogeochemical processes during early diagenesis [34]. In addition, the relationship between the phosphorus cycle and other biogeochemical cycles (for example, C and N) determines the important role of phosphate rock formation (phosphogenesis) in regulating the Earth's climate, as well as in regulating nitrogen and atmospheric oxygen levels on geological time scales [105-107].
Igneous apatite (rich in fluorine) is a ubiquitous accessory mineral found in almost all igneous rocks, from basic to acidic, although 0.11 vol.% of the rock is in a more normal range [108]. However, some special magmatic systems can often lead to economically valuable accumulations of apatite (concentrations > 3-5 vol.% of the rock) [34, 98-109]. These igneous phosphate accumulations are mainly associated with carbonatite/ alkaline systems (dominant) and/or some anorthosite magmas [35, 109]. The Khibiny and Kovdorsky alkaline-carbonatite complexes on the Kola Peninsula (Russia), where phosphate rocks mainly consist of either apatite-nepheline-rich rocks (Khibiny) or magnetite-apatite-rich ultramafic plutonic rocks (foscorites, Kovdor), form the world's largest igneous phosphate deposit [35, 110-114]. In addition to Russia, other important carbonatite/alkaline phosphate ores have been recorded and mined, for example, in Palabora in South Africa, Siilinarvi in Finland, Yakupiranga in Brazil, and Dorowa in Zimbabwe—mainly for fertilizer production [34, 35, 115].
Technogenic Mineral Formations (TMF) in the form of phosphate-siliceous shales were formed during the development of the Zhanatas, Kokjon, Kistas, and Tiesai phosphorite deposits. The ore extracted from the quarries is shipped to processing plants. Man-made mineral formations (flints, phosphate-siliceous shales, and substandard ores) are stored in special landfills.
Ore deposits of varying quality and man-made mineral formations from special dumps are combined in factories for the production of crushed phosphate raw materials [116, 117].
- The flint dump of the Zhanatas phosphorite deposit is intended for storing high-silica ores, the actual reserves of which in 2019 amount to 3804.18 thousand tons, with an average content of P2O5 -2.79%, SiO2 - 79.8%.
- The dump of phosphate-siliceous shales of the Kokjon phosphorite deposit, the Kistas site in 2019, amounts to 1630.14 thousand tons, with an average content of P2O5 -11.2%, SiO2 -52.4%. The dump is situated on a gentle slope with an absolute elevation difference of 10-15 m within its boundaries, with an actual length of 230 m at the bottom.
- The dump of substandard ores at the Tiesai phosphorite deposit for 2019 is 9412.8 thousand tons, with an average content of P2O5 -16.1%, SiO2 - 27%.
The influence of disturbed lands on local factors is evident in atmospheric air pollution resulting from work and vehicle traffic, as well as groundwater pollution in the mining area. In areas where production facilities are located, the inhabitants of the animal world are displaced outside the enterprise’s territory. Vegetation on the sites of facilities is destroyed for the period of operation, and its restoration is possible only after the complete elimination of facilities and the completion of reclamation work.
These dumps began to form in different periods. Data on the time of their formation and the enterprises that formed them are shown in Table 3.
Since 1999, the above-mentioned dumps have been part of Kazphosphate LLP. The landscape of the solid mineral waste dump sites is shown in Figs. (5-7).
| Name of the dump | Dump formation quarry |
Beginning of formation, year |
End of formation, year |
The content of the main components, % | Actual reserves, thousand tons | |
|---|---|---|---|---|---|---|
| P2O5 | SiO2 | |||||
| Flint dumps No. 4, No. 4A of the Zhanatas phosphorite deposit | Central block 3 | 1974 | 1993 | 2.79 | 79.8 | 2879.7 |
| Block 4 | 2.6 | 78.1 | 924.48 | |||
| Phosphate-siliceous shale dump of the Kokjon phosphorite deposit, Kista site | Kista block 1 | 1977 | 1992 | 11.2 | 46.1 | 743.8 |
| Kista block 2 | 11.94 | 52.4 | 886.34 | |||
| Dumps of substandard ores No. 3, No. 4, and No. 5 of the Tiesai phosphorite deposit | Block 3 | 1978 | 1993 | 16.1 | 3.4 | 2386.8 |
| Block 4 | 16.1 | 26.1 | 4326.0 | |||
| Block 5 | 16.1 | 27.0 | 2700.0 | |||



According to the international nomenclature of maps, the dump of phosphate-siliceous shales of the Zhanatas deposit of the Karatau Phosphorite-Bearing Basin (KPBB) is located in the area of sheet K-42-20 [118]. They have a limited distribution, forming a narrow band (up to 300 meters) in the Central part of the deposit. Lithologically, the rocks are represented (from bottom to top) by gray dolomites, characterized by a tiled texture and a fine-grained structure, alternating above with cherry-red or green siliceous-clay shales and siltstones (Fig. 8).
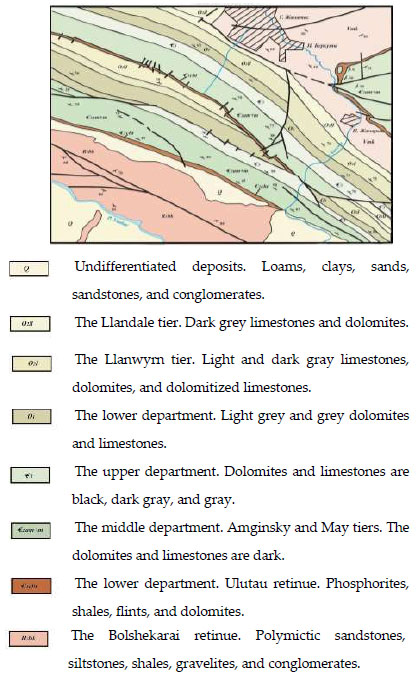
Geological map of the area, Scale 1:100,000 [118].
The Zhanatas deposit is represented by two industrial phosphorite strata and separating rock strata of the phosphate-shale pack. The peculiarity of the structure of the phosphatic siliceous shale dumps is due to their technogenic nature of formation. All the rocks of the internal overburden of the phosphate-shale pack were stored in the dump in the form of Technogenic Mineral Formations (TMF), which were formed at the site of the Zhanatas phosphorite deposit. The phosphate-siliceous shale dump is located on the territory of the Sarysu district in the Zhambyl region, at a distance of 10-12 km west of the city of Zhanatas Table 4, and Fig. 9).
Technogenic-mineral formations are homogeneous in their material composition and are characterized by a low content of valuable components. According to their physical and mechanical properties, they are represented by fragmented rocks, clastic material of various sizes. The parameters of the phosphate-siliceous shale dump of the Zhanatas deposit and the characteristics of the TMF, as well as the average composition, are shown in Fig. (10) and Table 5-7.
| Corner points |
Coordinates of the corner points |
|
|---|---|---|
| Northern latitude | Eastern longitude | |
| 1 | 43°33'22.02” | 69°37'58.45” |
| 2 | 43°33'23.66” | 69°37'52.24” |
| 3 | 43°33'28.48” | 69°37'48.26” |
| 4 | 43°33'35.15” | 69°37'45.92” |
| 5 | 43°33'41.50” | 69°37'47.80” |
| 6 | 43°33'42.49” | 69°38'0.31” |
| 7 | 43°33'37.98” | 69°38'4.45” |
| 8 | 43°33'32.74” | 69°38'6.52” |
| 9 | 43°33'27.71” | 69°38'6.05” |
| 10 | 43°33'23.51” | 69°38'4.36” |
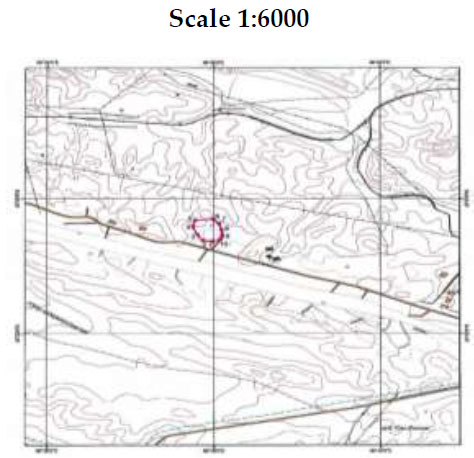
Cartogram of the location of the mining branch of the TMF dump No. 3 of the Zhanatas phosphorite deposit [118].
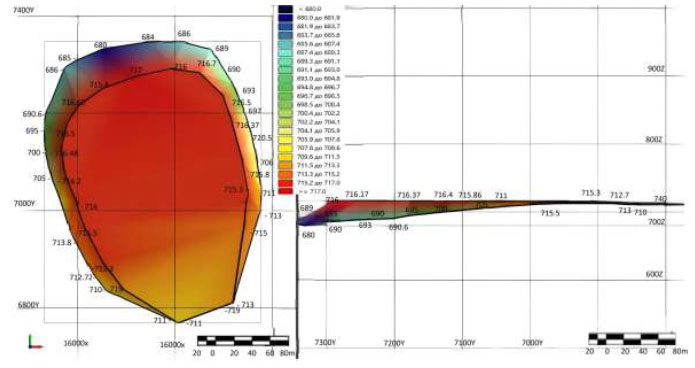
Parameters of the TMF dump [118].
| Parameters |
Units of measurement |
Inventory category |
|---|---|---|
| С1 | ||
| Phosphate-siliceous shales | Tonne | 4139214 |
| Average content of P2O5 | % | 14.2 |
| Average content of MgO | % | 2.1 |
The figure shows the phosphate of siliceous shales in axonometric view of the Zhanatas deposit. Rich in siliceous composition of 72.8% and the main component of P2O5 up to 11.2% corresponding to a width of 711.5-716.7 m and a length on a scaled scale from 7000 to 7300 m. In color, it corresponds to a dark red.
| Dump | Average component contents, % | |||
|---|---|---|---|---|
| P2O5 | MgO | R2O3 | CO2 | |
| Phosphate-siliceous shales | 14.2 | 2.1 | 6.1 | 4.7 |
Many researchers have proposed using off-balance phosphorites (P2O5 15.0-20.0%), phosphatized silicones (P2O5 1.0-8.0%), and shale phosphorites (P2O5 8.0-15.0%) in phosphorus production, the total resources of which are estimated to be in the thousands of tons within the calculation contours of balance reserves. If two products (phosphorite and quartz) used in certain proportions for the preparation of a charge are considered conditioned at the given normalized contents of individual components, then the mixture of these products should also be considered conditioned. The parameters characterizing the raw materials should be universal, e.g., an assessment of the suitability for electric distillation of the entire lithological range of phosphate-siliceous raw materials. In this regard, the technical conditions should allow the use of siliceous rocks of the Karatau basin instead of standard quartzites [117, 119, 120].
| The name of the rocks composing the base of the dump |
Hardness on the Protodiakonov scale |
Humidity, % | Volumetric weight, g/cm3 | Density, g/cm3 |
|---|---|---|---|---|
| Dolomites, dolomite- formed limestones |
12-14 | 1.0 | 2.65 | 2.84 |
6. EFFECTIVE DIRECTIONS OF AGGLOMERATION OF PHOSPHATE RAW MATERIALS
Thermal enrichment has become widespread in the world practice of phosphate ore processing. Roasting is primarily used for refining phosphate ores in the USA and North Africa. The process is carried out in fluidized bed furnaces, with three-stage furnaces being the predominant type.
During the heat treatment of phosphate raw materials, reactions occur in the solid phase between CaCO3, MdCO3, CaO, and MdO, as well as oxides of SiO2, Al2O3, Fe2O3 contained in the ore or concentrate. At high temperatures (1000°C, 1200°C, and above), the mobility of ions and cations in the lattice of crystals of these substances increases. Silicates, aluminates, and calcium ferrites are formed; therefore, the composition of the products of roasting raw materials usually includes bicalcium silicates, monocalcium aluminate, and bicalcium ferrite. When choosing the technology for processing phosphorite ores, the following provisions are taken into account, based on current global experience in ore processing at similar deposits: Dry processing methods are simpler and less expensive compared to wet processes. To date, dry methods have been widely used in large processing plants. Dry enrichment schemes include operations such as selective crushing and disintegration, screening, dedusting, and calcining [4, 41].
The use of low-grade phosphate raw materials in the electrothermal production of phosphorus necessitates improvements in sintering. In this direction, the authors propose using Phosphate-Siliceous Shales (PSS) from the Zhanatassky deposit as a fluxing component with partial replacement of the fuel component with oil refining waste, which contributes to an increase in the charge composition of the main component and a reduction in production costs [120].
The assessment of the suitability of the PSS was calculated based on the value of the P2O5 /CaO or P2O5 /CaO+MgO ratio. Theoretically, this ratio, based on the composition of phosphate raw materials, is 0.845, while the P2O5 content ranges from 29% to 31%, depending on the Mk. For Karatau phosphorites, shales, and off-balance phosphorites, this ratio ranges from 0.46 (more often 0.5) to 0.58 (rarely 0.6). The Mk for these phosphorites ranges from 0.36 to 0.46; therefore, the introduction of phosphate-siliceous rocks into the charge, to achieve the required Mk = 0.8, will lead to an increase in P2O5 in the charge.
The granulometric composition of the starting materials, determined using sieve analysis, is shown in Table 8.
As can be seen from the table, conditioned phosphorite contains about 39% of the fraction with a grain size of +5.0 mm. Shales mainly consist of a fraction of +5.0 mm.
Table 9 presents the volume weight of the starting materials of various fractional compositions.
From the presented data on the chemical composition of the most characteristic samples of lump phosphorite and phosphorus fines, it follows that the content of P2O5 in phosphorus fines is somewhat lower.
The increased content of alumina in phosphorite fines is explained by the fact that alumina minerals contained in the host rocks, which have a lower strength, pass through clay screening during crushing and grinding, enriching it with aluminum oxide.
| No. | Name of materials | Fraction, mm | ||||
|---|---|---|---|---|---|---|
| +5.0 | +3.0 | +1.0 | +0.5 | +0.1 | ||
| 1 | Сonditioned зhosphorite | 38.7 | 20.5 | 15.01 | 10.17 | 7.03 |
| 2 | Phosphate-siliceous shales | 50.8 | 16.03 | 12.60 | 8.72 | 4.53 |
| 3 | Clay phosphate-siliceous shales | 48.1 | 15.9 | 10.25 | 9.1 | 7.61 |
| 4 | Coke | 38.2 | 24.7 | 19.30 | 9.61 | 3.35 |
Tleuov et al. [121, 122] proposed an environmentally efficient method for agglomeration roasting of low-grade phosphorite ores and phosphate-rolled shales from the Zhanatas deposit using oil sludge as a fuel component.
| No. | Name of materials | Fraction, mm | ||||
|---|---|---|---|---|---|---|
| +5.0 | +3.0 | +1.0 | +0.5 | +0.1 | ||
| 1 | High-quality phosphorite |
|
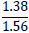
|
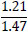
|
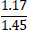
|
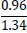
|
| 2 | Shale: a) Phosphate- siliceous shale (black) |
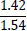
|
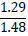
|
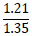
|
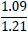
|
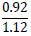
|
| 3 | b) clay phosphatosilicate |
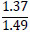
|
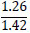
|
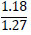
|
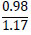
|
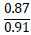
|
| 4 | Сoke |
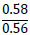
|
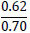
|
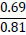
|
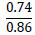
|
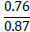
|
The characteristics of the composition and phase structures of phosphate-siliceous shales from the Zhanatas deposit were studied to understand the physico-chemical features of the proposed agglomeration method (Fig. 11).
The spectra showed the intensities of the characteristic X-ray lines for the elements present in the sample. Peaks corresponding to the main and secondary elements are visible on the spectrum. Key peaks include Si-Kα, P-Kα, Ca-Kα, Fe-Kα, and Zr-Lα, which confirm the presence of silica, phosphates, calcium, iron, and zirconium in the sample. The presence of peaks corresponding to trace elements, such as Cr-Kα, Mn-Kα, and Ti-Kα, indicates low concentrations of these elements.
Basic oxide SiO2 (26.2%) characterizes the siliceous nature of the shale. The content of calcium oxide CaO reaches 37.8%, which indicates the presence of calcite or apatite minerals. The high phosphorus content of P2O5 (13.9%) is typical for phosphate rocks.
Impurity oxides MgO (3.00%), Al2O3 (4.72%), and Fe2O3 (2.25%), in smaller quantities, indicate mineralogical diversity. Trace amounts of heavy metals, such as Cr2O3 (0.0511%), TiO2 (0.240%), and ZrO2 (0.222%), are also present in the sample. Small amounts of rare earth elements, such as Eu2O3 (0.0616%) and Dy2O3 (0.0189%), are of industrial importance.
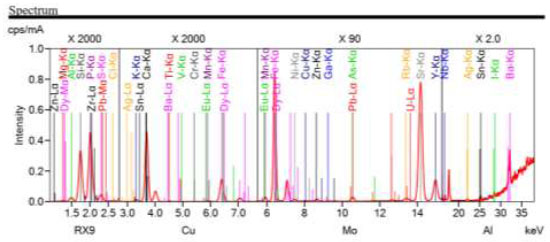
Spectral analysis of phosphate-siliceous shale allowed us to determine the chemical composition of the sample, both the main components and the impurity elements. The results showed that the bulk of the material consists of silica (SiO2) and calcium oxide (CaO), which is typical for this type of rock. The significant content of P2O5 confirms that the rock is a valuable source of phosphates, which can be useful in its application and processing methods.
A distinctive feature of the technological scheme for producing yellow phosphorus was the formation of phosphorous ore fines with a size of 10-0 mm, formed during the extraction and production of commercial lump ore, which were processed at previously operating phosphorus enterprises. Phosphorite fines, the yield of which reached 40-48% of the phosphate raw materials extracted from the bowels, had not previously been used in phosphorus production and were stored in mine dumps, and the funds spent on extracting this mass of ore were frozen.
Studies by S. Shumakov and Pekhotin [123], have shown the effectiveness of agglomeration roasting of phosphorous fines for phosphate ores of the Karatau deposit. The agglomeration received small fractions of phosphorites from the current production from the Zhanatas mine, which were heterogeneous in both chemical and granulometric composition. Coke fines were sifted from the drying department and the charge department of the furnace shop as sintering fuel. When using non-averaged cokes (Karagandinsky and Kuznetsky), which significantly differ in ash, carbon, and sulfur content, the amount of carbon in the charge varied from 1.8 to 10.8%. The moisture content of the charge in the first period of operation of the sintering plant ranged from 3 to 12%. The lack of a dosage of hot and cold return made it impossible to maintain a constant charge composition. During the survey period, the total refund amount varied from 42% to 60%. This led to an uncontrollable change in the amount of carbon in the charge from 3.02 to 7.82%, which, in turn, affected the process parameters (including the return yield), causing a large range of fluctuations. The content of CO2 in the initial charge, according to the average daily analyses, varied from 2.41 to 3.73% with an average value of 2.89%. The average amount of carbon in it was 5.29%.
Tleuov et al. [117] studied the change in the sieve composition of the agglomerate and the formation of fines (return) along the entire path of sintering and feeding the finished product from the sinter machine to the dispensers of the furnace shop. Studies have shown that pieces of agglomerate are destroyed to form small fractions. The cold return yield averaged 21.96% of the mass of the sinter. During the control screening, an additional 9.0% of the fines were allocated. In addition, approximately 8.7% of the sinter’s mass was returned to the sintering process as a “bed”. Taking this into account, the yield of suitable agglomerate was 40.05% [124].
Meshalkin et al. [125] presented the construction of an intelligent control system for a complex chemical and energy technology process in their study for the preparation of phosphate raw materials (phosphorite pellets) in multi-chamber conveyor-type roasting machines for the further production of phosphorus and phosphorus-containing compounds.
The system manages the pellet production process, ensuring the optimization of the chemical and energy technology system units according to the criterion of minimizing energy and resource consumption.
A proposed pyrometallurgical phosphorite processing technology has the potential to significantly address the issues of energy-resource-efficient processing of phosphorite mineral raw materials that the industry has been facing for many years [126, 127]. The initial ore or concentrate, depending on its CaO and Al2O3 content, is mixed with silicon-alumina containing raw materials (possibly waste) to ensure the viscosity of the melt. Subsequent melting in electroslag mode enables the production of phosphoric anhydride, ammonium fluoride, metal ligature, and high-quality cement clinker.
Thermal enrichment (roasting) has become a widespread practice in the global phosphate ore processing industry. Roasting is primarily used for refining phosphate ores in the USA and North Africa. The process is carried out in furnaces, among which three-stage furnaces are the predominant type. Ores (concentrates) containing calcite, magnesite, and siderite, subjected to thermal exposure at a temperature of 850-1100°C, decompose with the release of oxides of calcium, magnesium, and iron during subsequent processing. During the firing of raw materials, reactions occur in the solid phase between CaCO3, MdCO3, CaO, and MdO, as well as oxides of SiO2, Al2O3, Fe2O3 contained in the ore or concentrate. At high temperatures (1000°C, 1200°C, and above), silicates, aluminates, and calcium ferrites are formed. When choosing the technology for thermal processing of phosphorite ores, the following provisions were taken into account, based on the current global experience with ore processing in similar deposits. Dry enrichment schemes include operations such as selective crushing and disintegration, screening, dedusting, and thermal firing [128, 129].
In our proposed technology, agglomeration sintering of a charge consisting of low-grade phosphorites and PSS is carried out on a moving grate with air being sucked through the charge layer [130]. The high temperature of the process (up to 1623 K) is achieved due to the combustion of solid fuel (coke fines or petroleum coke), which is part of the sintering mix (Fig. 12).
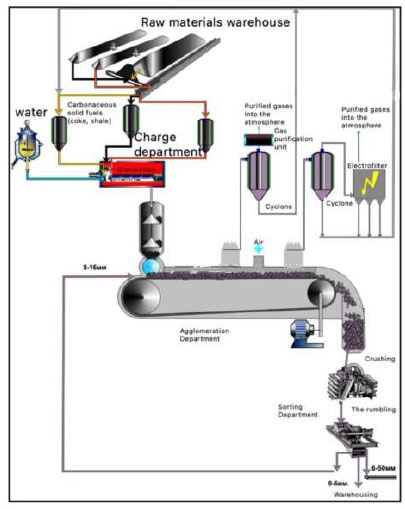
Technological scheme of agglomeration of phosphorous fines [130].
The ignition of the fuel component on the surface of the layer occurs in a furnace, based on natural (CH4) and furnace gas (CO). As the sintered charge moves towards the unloading part of the sintering machine, combustion, which began from the surface of the charge layer, sequentially passes through the entire thickness of the material and ends at the grate. Ignition of solid fuel in the layer occurs at a temperature of T = 973 K. The solid fuel of the charge burns almost completely, and the ore fines are melted and sintered, forming a porous “pie”, which, after crushing, yields a lumped product – an agglomerate. The suction of air necessary for the oxidation of carbon of solid fuels is carried out by exhusters installed on the path of agglomeration gases.
The main physico-chemical transformations occur in the melting zone and intensive heating. The chemical compounds formed during these processes are characterized by the formation of an offlussed phosphorite agglomerate, while the degree of decarbonization of phosphorite reaches 95%.
In order to protect the environment from harmful gaseous emissions, a closed cycle of waste gas regeneration and the return of an unusable fraction of the agglomerate to the process head is employed.
CONCLUSION
The presented article provides a comprehensive and well-founded analysis of the current state of the world's phosphate reserves in the context of recent generations. Special attention is paid to the challenges of processing phosphate raw materials, including low-grade and substandard ores. The main technological approaches to chemical and mechanochemical methods of enrichment, as well as acid extraction and electrofluoric melting, are described in detail.
The problems of depleting rich phosphate resources and increasing the innovativeness of processing low-grade phosphate ores and industrial waste were highlighted. The analyzed studies have shown that the use of mechanical and thermal methods for processing phosphorous ores will increase the economic efficiency of phosphorus production.
Special attention is paid to the significant industrial fields of Karatau and Chilisai, which have high potential for industrial development. A comparison of modern technologies for processing phosphate raw materials applicable to phosphate-siliceous shales provides grounds for identifying the most promising areas for further technological processing.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: S.A.: Writing - Original Draft Preparation; Z.T., N.S.: Data Collection; D.P.: Methodology; A.T.: Writing - Reviewing and Editing; S.P.: Investigation; M.U.: Data Analysis or Interpretation;.
LIST OF ABBREVIATIONS
| LLP | = Limited Liability Partnership |
| CIS | = Commonwealth of Independent States |
| USA | = United States of America |
| JSC | = Joint Stock Company |
| MPP | = Mining and processing plant |
| CFE | = Chemical flotation enrichment |
| MAP | = Monoammonium phosphates |
| NDPP | = Novodzhambul Phosphorous Plant |
| TPA | = Thermal phosphoric acid |
| FG | = Food grade |
| PP | = Polpinskoye deposit |
| KyzP | = Kyzylkumskoye deposit |
| KSP | = Koksu deposit |
| KJP | = Kokjon deposit |
| KHAK | = Khibinsky deposit |
| PS | = Phosphorous sludge |
| CM | = Cottrell milk |
| REE | = Rare earth elements |
| TMF | = Technogenic mineral formations |
| KPBB | = Karatau phosphorite-bearing basin |
| PSS | = Phosphate-siliceous shales |
AVAILABILITY OF DATA AND MATERIALS
The data sets used and/or analysed during this study are available from the corresponding author [A.T.] upon request.
FUNDING
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number BR21882181 “Development of technology for production of high-efficiency materials based on mineral raw materials and anthropogenic wastes”.
ACKNOWLEDGEMENTS
Declared none.