All published articles of this journal are available on ScienceDirect.
Recovery of Tantalum from a Semi-Product of the Comprehensive Processing of Waste from a Rhenium-Containing Nickel-Based Superalloy
Abstract
Introduction
To address pressing resource and environmental challenges, this study investigates the tantalum recovery from a Ta-W semi-product generated during the processing of grinding waste from the ZhS32 VI rhenium-nickel superalloy.
Methods
The Ta-W semi-product was sintered with NaOH (700–1000 °C) to oxidize TaC into insoluble sodium tantalates (NaTaO3/Na5TaO5), with an optimal ratio (semi-product: NaOH) and temperature determined. The sintered residue underwent acid leaching (HF/H2SO4 mixture, room temperature), where leaching parameters (time, phase ratio) were optimized; the kinetics was studied, and K2TaF7 was precipitated from the leachate using KCl.
Results
Minimum Ta extraction into solution during sodium hydroxide sinter leaching, along with complete transfer of W and Mo to the aqueous phase, is achieved at 700°C and a 1:3 (g/g) ratio. Subsequent leaching of the solid residue with an HF-H2SO4 mixture (1 g: 1 mL: 1 mL) enables complete Ta recovery (>99.99%) within 30 minutes. The kinetics of tantalum acid leaching from the residue indicates a shift in the rate-limiting step from diffusion to chemical reaction. Through precipitation from the sulfate-fluoride solution using a KCl solution, Ta was obtained as K2TaF7 (~52.64% Ta).
Discussion
The presented process allows for the selective separation of tantalum from tungsten and molybdenum. The kinetics show a transition from diffusion to reaction, which enables efficient extraction at room temperature. Controlling impurities in K2TaF7 requires further refinement.
Conclusion
An effective two-stage hydrometallurgical process (alkaline sintering + acid leaching) enables high-yield tantalum recovery from superalloy grinding waste while facilitating valuable component recycling from heat-resistant alloy residues.
1. INTRODUCTION
The problem of waste utilization and processing has acquired a global concern, becoming one of the key challenges for the sustainable development of modern technologies [1, 2]. Special significance is given to the recycling of waste containing rare and non-ferrous metals, whose reserves are limited and demand is constantly growing due to their use in high-tech industries: aerospace, electronics, nuclear energy, and medicine [3-5]. Among these critically important metals is tantalum [6, 7].
Tantalum possesses a unique combination of properties: a high melting point (3017 °C), exceptional corrosion resistance (close to noble metals), good manufacturability, high thermal conductivity, and the ability to form stable anodic films [8, 9]. These properties make it indispensable in the production of supercapacitors and miniature capacitors. Tantalum powders and anode wire form the basis for electrolytic capacitors with high specific capacitance, stability, and reliability, which are used in almost all portable electronics, telecommunications equipment, and control systems [10-12]. Tantalum is also a key alloying element in nickel and cobalt superalloys for manufacturing heat-resistant alloys, such as turbine blades of gas turbines (aviation, power generation), combustion chambers, and other components operating under extreme temperature and load conditions [13-15]. Due to its high corrosion resistance in aggressive environments (acids, melts), tantalum is widely used as a structural material in chemical equipment, including heat exchangers, reactors, mixers, and pipelines in the chemical and pharmaceutical industries [16, 17]. Its biological inertness allows the use of tantalum for manufacturing medical implants, such as bone implants, surgical clips, and stents [18, 19]. Additionally, tantalum carbide (TaC) in hard alloys based on tungsten carbide (WC) significantly enhances their wear resistance, toughness, and thermal stability, making it a valuable component for cutting tools and drilling equipment [20, 21].
The primary source of tantalum is the columbite-tantalite mineral ((Fe, Mn)(Nb, Ta)2O6), but its deposits are limited and unevenly distributed geographically [22, 23]. A significant portion of the world’s tantalum production is associated with the processing of tin slags [24]. Growing demand and limited primary resources make the development of recovery technologies from secondary raw materials critically important [25-27].
Of particular interest is waste from the production and operation of heat-resistant rhenium-nickel superalloys (such as ZhS32 VI, mentioned in the study). These alloys contain a complex set of valuable metals: nickel, cobalt, chromium, rhenium, tungsten, molybdenum, aluminum, titanium, tantalum, hafnium, zirconium, and rare earth elements [28-30]. Traditional recycling technologies for such complex multi-component waste include pyrometallurgical (smelting, chlorination) and hydrometallurgical (acidic or alkaline leaching) methods, or their combinations [31-33]. Currently, there is no information on the recovery of tantalum from such products.
In the presented research, the starting raw material is the Ta-W semi-product, formed during one stage of the acid processing of rhenium-nickel superalloy grinding waste. It represents a complex mixture of oxides, carbides, and metals, with tantalum and tungsten as the main valuable components, and impurities including titanium, silicon, zirconium, aluminum, and traces of other elements [34]. The task of recovering tantalum from such a matrix is complicated by its chemical inertness, as its compounds (especially Ta2O5 oxide and TaC carbide) exhibit high resistance to most acids and alkalis [35, 36], as well as the complex phase composition of the semi-product itself, which is heterogeneous in both composition and structure [37]. Additional difficulties arise from the presence of interfering elements such as titanium, zirconium, silicon, and tungsten, which have similar chemical properties to tantalum. These elements complicate selective recovery [38-40] and the need to obtain a commercial product suitable for further use [41].
Traditional approaches to the dissolution of tantalum-containing materials include acid decomposition using HF mixtures with HNO3 and H2SO4 [42], alkaline fusion/sintering with NaOH or KOH at high temperatures to form insoluble or soluble tantalates [43, 44], and interaction with chlorine or chlorinating agents (Cl2, CCl4, CO+Cl2) at elevated temperatures to produce volatile chlorides [45]. The choice of method for a specific raw material depends on its composition, the required purity of the product, and economic feasibility [46].
To address these problems, the presented study focuses on an efficient and selective two-stage hydrometallurgical scheme. It includes preliminary alkaline sintering to convert tantalum into a chemically active form (tantalate) [47], followed by acid leaching with a mixture of hydrofluoric and sulfuric acids to transfer it into solution [48, 49]. Particular attention is paid to optimizing the parameters of each stage and studying the kinetics and mechanism of acid leaching [50-52].
2. MATERIALS AND METHODS
2.1. Reagents and Equipment
In the course of tantalum extraction from the Ta-W-semi-product, the following reagents were used: NaOH (ACS), H2SO4 (96 wt. %) (ACS), and HF (40−42 wt. %) (ACS). A suite of equipment was used for analyzing the solid phase and solutions: an optical emission spectrometer with inductively coupled plasma (Optima 4300 DV, Perkin Elmer, USA), a mass spectrometer with inductively coupled plasma (XSeries ICP-MS, Thermo Scientific Inc., USA), an X-ray diffractometer XRD 7000, Shimadzu, Japan, and a scanning electron microscope with a micro-X-ray spectrometer (JSM-5900LV, JEOL, Japan) for microanalysis and studying the morphology of the obtained concentrate.
2.2. Determination of Physicochemical Characteristics of Ta-W-Semi-Product
The elemental composition of the initial Ta-W-semi-product, determined by ICP-MS, is presented in Table 1.
Table 1.
| Fraction, mm | Content, wt. % | ||||||
|---|---|---|---|---|---|---|---|
| Ta | W | Mo | Si | Ti | Zr | Al | |
| +0,5 | 8,92 | 10,32 | 5,21 | 3,19 | 0,22 | 5,24 | 18,99 |
| -0,5 +0,2 | 13,35 | 16,40 | 4,34 | 0,12 | 1,75 | 0,29 | 17,65 |
| -0,2 +0,1 | 17,94 | 14,58 | 1,17 | 1,51 | 4,44 | 2,14 | 6,79 |
| -0,1 | 15,37 | 11,00 | 3,98 | 0,59 | 5,58 | 0,18 | 10,20 |
As shown in Table 1, the elements are present in all fractions; therefore, subsequent experiments were conducted on an averaged sample ground in an agate mortar to a particle size of -0.1 mm.
The moisture content of the semi-product was determined using two samples, each weighing 150 g. The samples were dried in a muffle furnace to constant mass at the following regime: heating rate of 15°C/min, isothermal hold at 90±5°C, duration for 8 h. The average yield of dry cake was 75% of the initial, and the moisture content of the product was 25%.
2.3. Sintering of Ta-W Semi-Product with Sodium Hydroxide
The sintering of the Ta-W semi-finished product with NaOH was carried out by thoroughly mixing the initial semi-finished product with granular sodium hydroxide in mass ratios of 1:2, 1:4, 1:6, 1:8, and 1:10 (g:g). The mixture was loaded into corundum crucibles and sintered in a muffle furnace (SNOL 10/11) in an air-oxygen environment at temperatures of 700, 800, 900, and 1000 °C respectively.
For the main experiments aimed at optimizing the phase ratio and temperature, the following regime was selected − heating to the sintering temperature over 1 h and holding at that temperature for 1 h.
The purpose of the sintering was to convert tantalum compounds (TaC and Ta2O5) into insoluble sodium tantalates (NaTaO3 and Na5TaO5) for subsequent separation from soluble components (Na2WO4, Na2MoO4), which is described by the oxidation reactions of tantalum carbide to pentoxide and its interaction with sodium hydroxide:
4TaC + 9O2 → 2Ta2O5 + 4CO2
Ta2O5 + 2NaOH → 2NaTaO3 + H2O
Ta2O5 + 10NaOH → 2Na5TaO5 + 5H2O
2.4. Aqueous Leaching of the Sintered Ta-W Semi-Product with Sodium Hydroxide
After cooling, the sintered material was subjected to aqueous leaching with distilled water in a thermostatic cell, using an overhead stirrer to stir at a fixed phase ratio of sintered material to water of 1:10 (g: mL). The main task of this stage was to dissolve tungsten and molybdenum compounds.
The tantalum, tungsten, and molybdenum contents in the solutions and solid residue were determined by ICP-MS. For this purpose, sample preparation was carried out in a microwave system for acid digestion of samples (MARS 6, CEM) using a mixture of nitric and hydrofluoric acids. Сalculating the recovery degree of elements into the liquid phase during water leaching relative to the total element content in the initial semi-product (Eqs. 1−3):
αTa = (mTa / mTa0) ∙ 100%
where mTa – the mass of tantalum at the current time in the solution, g;
mTa0 – the initial mass of tantalum in the sample of the semi-finished product, g;
αW = (mW / mW0) ∙ 100%
mW – the mass of tungsten at the current time in the solution, g;
mW0 – the initial mass of tungsten in the sample of the semi-finished product, g
αMo = (mMo / mMo0) ∙ 100%
mMo – the mass of molybdenum at the current time in the solution, g;
mMo0 – the initial mass of molybdenum in the sample of the semi-finished product, g.
2.5. Leaching of Tantalum from Ta Semi-Finished Product with a Mixture of Concentrated Hydrofluoric and Sulfuric Acids
The solid residue containing sodium tantalate, impurities of titanium, silicon, and zirconium, was subjected to acid leaching with a mixture of concentrated hydrofluoric (40−42 wt. %) and sulfuric (96 wt. %) acids. The process was carried out at room temperature (22±2°C) in fluoroplastic beakers with stirring, exploring phase ratios of Ta semi-finished product (g): HF (mL): H2SO4 (mL) – 1: 1: 1, 1: 1: 2, 1: 1: 3, 1: 2: 1, and 1: 3: 1.
To study the kinetics of acid leaching at a ratio of Ta: solution of 1: 1: 1 (g: mL: mL), aliquots of the solution were taken after 5, 10, 15, 25, and 30 minutes and analyzed for Ta content using ICP-MS, calculating the degree of Ta extraction into the solution (αTa, wt.%) according to Eq. 1.
2.6. Mathematical Processing of the Integral Kinetic Curve of Tantalum Leaching from Ta Semi-Finished Product with a Mixture of Concentrated Hydrofluoric and Sulfuric Acids
To determine the limiting stage of the acid leaching process, experimental kinetic data were approximated using equations from 12 different kinetic and diffusion models [53, 54] (Table 2, equations 4−15).
2.7. Precipitation of Tantalum Concentrate
In the final stage, the precipitation of K2TaF7 was carried out from a tantalum-containing sulfate-fluoride solution. The solution was heated to 80°C, and a hot solution of KCl (300 g/L) was slowly added to it at the same temperature with stirring, which led to the reaction [55]:
H2[TaF7] + 2KCl → K2TaF7↓ + 2HCl
The mixture was cooled and kept for 24 h to allow crystallization. The precipitate of K2TaF7 was filtered under vacuum, washed with distilled water (ratio S: L – 1: 3 (g: mL), three times), and dried in a muffle furnace to constant weight under the following conditions: heating rate – 15 °C/min, isothermal hold at 90±5°C, duration – 24 h.
The composition of the obtained product was analyzed using an electron microscope equipped with a micro-X-ray spectrometer (JSM-5900LV, JEOL, Japan).
3. RESULTS AND DISCUSSIONS
3.1. Alkaline Roasting Conditions
The influence of the ratio of Ta-W-semi-product phases to NaOH (g: g) and the roasting temperature on the tantalum extraction degree (αTa, wt. %) into solution during subsequent water leaching is shown in Fig. (1).
The maximum tantalum extraction degree, calculated by equation 1, is observed in the solution during water leaching when the roasted product is leached at a roasting temperature of 1000 °C, with a phase ratio of semi-product to sodium hydroxide of 1:12 (g:g), reaching 2.8 wt.%. An increase in αTa with growing phase ratio and temperature is associated with the formation of a water-soluble compound in strongly alkaline media − 7Na2O∙5Ta2O5 [56].
Thus, it has been established that during the leaching process of the alkaline roast, tantalum losses into the solution do not exceed 3 wt. %. The main objectives of the experiments were to convert tantalum into more reactive forms and to remove the accompanying macro-components W and Mo from the system. It has been determined that at a temperature of 700 °C and a phase ratio of semi-product to alkali 1:2 (g:g), the extraction degree of tungsten and molybdenum calculated by equations 2−3 into the aqueous phase exceeds 99.9 wt. % (Fig. 2), and therefore, it was concluded that these roasting conditions are optimal for removing these elements.
To confirm the conversion of tantalum into the NaTaO3 form, the X-ray phase analysis spectrum of the solid residue formed after leaching the roasts under the selected conditions was recorded (Fig. 3).
The identification of the spectrum confirmed the absence of tantalum oxide and carbide phases in the solid residue. Additionally, no metallic phase was detected.
| Model name | Equation |
|---|---|
| Valensi model | (1-α)∙ln(1-α)+α = kVt (4) |
| Zhuravlev model | ((1-α)-1/3-1)2 = kZt (5) |
| Exponential model | ln(α) = ket (6) |
| Krieger-Ziegler model | (1-(1-α)-1/3)2 = kKZt (7) |
| First-order reaction kinetic model | ln(1/1-α) = k1t (8) |
| Jander model | (1-(1-α)1/3)2 = kJt (9) |
| Ginstling-Brounshtein model | 1-(2/3) ∙ α-(1-α)2/3 = kGBt (10) |
| Shrinking sphere model | 1-(1-α)1/3= kSSt (11) |
| Kozeev-Erofeev model | (-ln(1-α))1/3 = kKEt (12) |
| Shrinking cylinder model | 1-(1-α)1/2 = kSCτ (13) |
| Anti-Jander model | ((1+α)1/3-1)2 = kaJt (14) |
| Anti-Ginstling-Brounshtein model | 1-(2/3)∙α-(1+α)2/3 = kAGBt (15) |
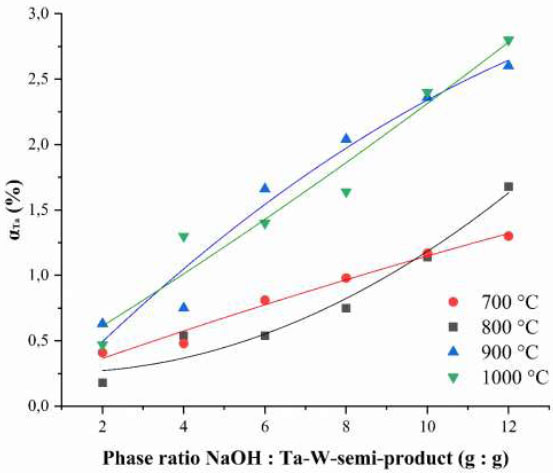
Dependence of the tantalum extraction degree from the roasted product into the leaching solution on the NaOH: Ta-W-semi-product phase ratio (G: G) during roasting.
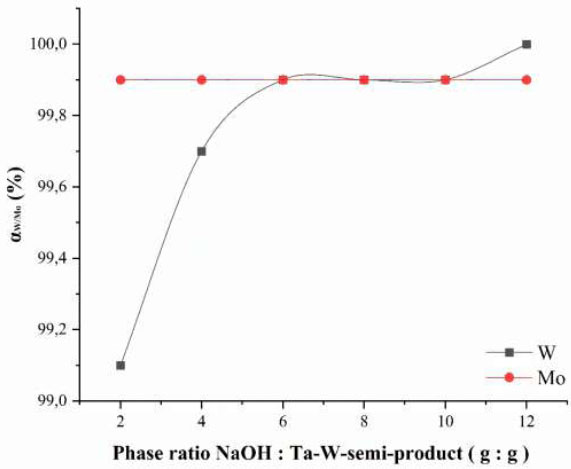
Dependence of the extraction degree of tungsten and molybdenum from the roast into the leaching solution on the phase ratio Naoh: Ta-W-semi-product (G: G) during roasting.
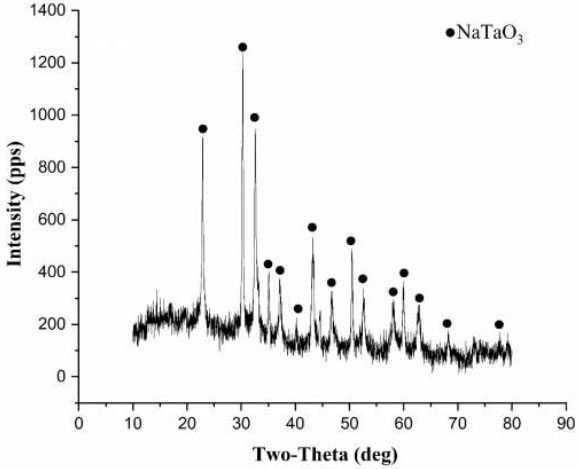
X-Ray diffraction pattern of the Ta-semi-product formed after leaching tungsten and molybdenum from the Ta-W intermediate product with alkalis.
3.2. Leaching of the Ta Semi-Product with a Mixture of Concentrated Hydrofluoric and Sulfuric Acids
An experiment was conducted to determine the optimal phase ratio: semi-product: HF: H2SO4 − g: ml: ml. The process duration was 8 hours. The results are presented in Fig. (4). In the first case, the ratio was varied by increasing the volume of sulfuric acid while keeping the amount of semi-product and hydrofluoric acid constant. In the second case, the volume of HF was changed while maintaining constant masses of the semi-product and sulfuric acid, respectively.
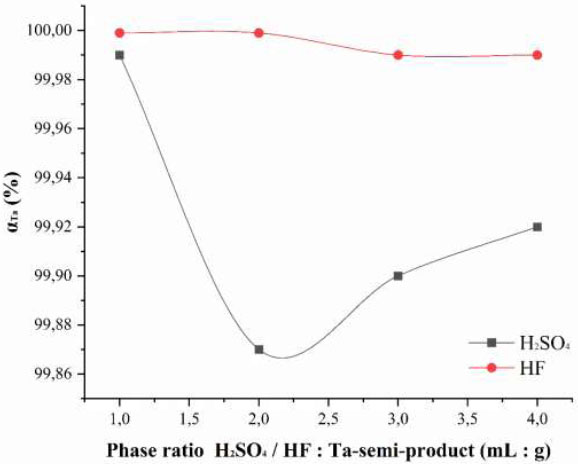
Dependence of the tantalum extraction degree from the speck into the leaching solution on the phase ratio naoh: Ta-W semi-product (G:G) during sintering.
Complete recovery of tantalum into the solution is achieved at the minimal phase ratio of Ta semi-product: HF: H2SO4 − 1: 1: 1 (g: mL: mL). Increasing the acid volumes does not improve the extraction efficiency. Using smaller acid volumes leads to the formation of filter-clogging pulps and complicates phase separation.
The kinetics of tantalum leaching from the Ta semi-product was studied (Fig. 5).
The kinetic curve has a characteristic shape with a rapid initial segment and a plateau at the level of complete extraction. The process proceeds at a high rate: approximately 94 wt. % Ta is extracted in 5 minutes, about 96 wt. % in 10 min, and nearly complete extraction (~99.99 wt. %) is achieved in 30 min.
To identify the rate-determining step of the tantalum leaching process, the kinetic curve was analyzed using 12 kinetic and diffusion models Table 2. The process is best described by the models: Jander, Gistling-Brounstein, and the first-order reaction kinetic model (Table 3 and Fig. 6).
The Jander and Gistling-Brounstein models (Fig. 6a and b) are characteristic of processes limited by diffusion through the reaction product layer formed around particles. The Jander model is a simplification of the Gistling-Brounstein model for low degrees of conversion.
A good fit with the first-order model (Fig. 6c) may indicate a significant contribution of the chemical reaction or a mixed regime at certain stages.
In the initial stages, the process is likely limited by the external diffusion of reagents (HF/H2SO4) to the surface of the semi-finished product particles. Then, as the reaction product layer (dissolved components) forms, diffusion of reagents through this layer to the unreacted core becomes limiting (Jander/Gistling-Brounstein models). At the final stage, when the product layer becomes porous or breaks down, the rate may again be controlled by the chemical reaction at the surface (first-order reaction kinetic model).
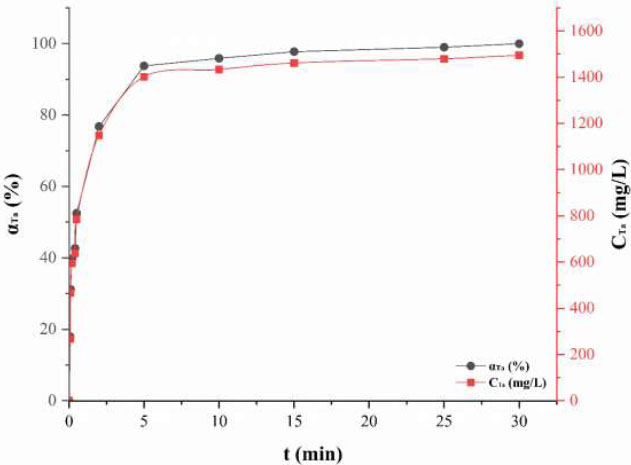
Integral kinetic curve of tantalum leaching from Ta semi-product (T − 22 ± 2 °C, phase ratio of semi-product: HF: H2SO4 – 1: 1: 1 G: Ml: Ml).
| Model name | k, min-1 | R2 |
|---|---|---|
| Ginstling-Brounshtein model | 0.042 | 0.9875 |
| Jander model | 0.072 | 0.9985 |
| First-Order Reaction Kinetic Model | 0.494 | 0.9888 |

Linearization of the kinetic curve in the models in coordinates: Gistling-Brounstein (A), Jander (B), and the First-Order Reaction Kinetic Equation (C).
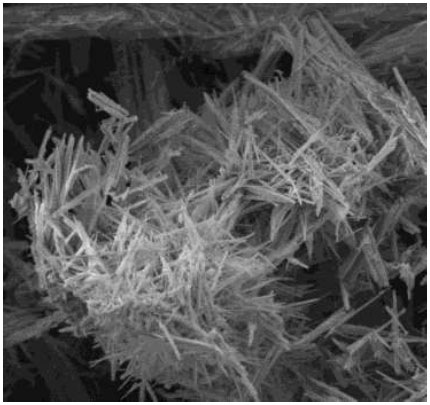
Micrograph of the precipitated potassium heptafluorotantalate.
3.3. Precipitation of Tantalum Concentrate
The sedimentation method for extracting tantalum using a potassium chloride solution (300 g/L) was tested, resulting in the concentration of potassium heptafluorotantalate (Fig. 7).
Based on the microphotograph of the product presented in Fig. (7), the following conclusions can be drawn:
- − Crystals exhibit needle-like/prismatic morphology with pronounced elongation along one axis. The typical length of the crystals is 1–3 µm with a width of 0.1–0.3 µm;
- − There is a chaotic orientation of crystals with a tendency to form loose agglomerates. Intercrystalline contacts are predominantly point contacts without signs of sintering.
- − A pronounced polydisperse character is observed, with particle size ratios up to 1:30. Predominantly nano- and microcrystals (50–300 nm) with inclusions of sub-micron needles;
- − Isometric particles (0.2–0.5 µm) of spheroidal shape are present. Defects in packing, such as screw dislocations, are observed.
- − Amorphous inclusions (light areas on the SE detector) are detected;
- − The morphology corresponds to hydrothermally precipitated K2TaF7 crystals formed under non-optimal crystallization conditions (polydispersity), with signs of growth inhibition along the (001) facets, and the presence of impurity phases (probably tantalum oxides or silicon fluorides).
The elemental composition of the product is presented in Table 4.
| The elements | Ta | K | S | Si | F | O |
| Content, wt. % | 52,64 | 11,8 | 1,81 | 3,54 | 19,9 | 11,0 |
The tantalum content in the obtained concentrate is 52.64 wt. %, which is higher than the theoretical value for K2TaF7 (46.14 wt. %), indicating the presence of impurities such as metal oxides and/or hydroxides in the precipitate. The high oxygen content of 11 wt. % confirms this.
The potassium content is 11.80 wt. %, which is lower than the theoretical value of 19.94 wt. %, also consistent with the presence of potassium-free tantalum compounds. The fluorine content is 19.90 wt. %, significantly below the theoretical 34.17 wt. %, further supporting the presence of non-fluoride forms of tantalum.
3.4. Technical and Economic Assessment of Recycling Technology
According to research [57], about 130,000 t of superalloy waste is generated annually worldwide. With an average tantalum content of ~ 4 wt. %, this results in 5.2 t of tantalum being obtained per year. The cost of 1 kg of tantalum (in terms of pentaoxide) in 2024 was USD 170 [58]. It is also worth considering that when recycling such waste, the greatest economic effect is brought by the extraction of rhenium. The content in the feedstock is ~ 4 wt. %, and according to Yu et al. [58], in 2024, the cost of 1 kg of rhenium is 1370 US dollars.
The use of alkaline sintering and subsequent acid leaching of the dry residue is employed in processing mineral raw materials, particularly tantalite-columbite. The main equipment used in scaling is: electric resistance furnaces, iron crucibles, apparatuses with a top-driven mixing device, fluoroplastic-lined [59-61].
CONCLUSION
The conducted research allowed the development and optimization of an effective hydrometallurgical scheme for extracting tantalum concentrate from a complex Ta-W semi-product formed during the processing of waste rhenium-nickel superalloy ZhS32 VI. It was established that, for maximum conversion of tantalum compounds into insoluble sodium tantalates and to ensure selectivity of subsequent aqueous leaching, optimal parameters are: temperature of 700 °C, mass ratio of Ta-W semi-product to NaOH of 1:2 (g:g), heating time of 1 h, and holding time of 1 hour. Under these conditions, the best separation of tantalum from tungsten and molybdenum, which are transferred into the solution, is achieved.
A highly efficient method for leaching tantalum from alkaline processing cake using a mixture of HF and H2SO4 at room temperature was developed. It was found that complete extraction of tantalum (>99.99 wt.%) is achieved with a minimal ratio of 1 g Ta semi-product: 1 ml HF (25 M): 1 ml H2SO4 (18 M) within 30 minutes. Increasing the amount of acids does not enhance the extraction process. In contrast, filtration problems can occur if we increase the amount of acids. The kinetics data of the process are characterized by a high rate, with 93.77 wt.% of Ta extracted within the first 5 minutes. Kinetic curves analysis using 12 mathematical models showed that the process is best described by the Jander diffusion model (R2 0.9985), the Gistling-Brownstein model (R2 0.9875), and a first-order reaction kinetic model (R2 0.9838), indicating a complex mechanism involving a change in rate-limiting stages. The initial stage (1–3 minutes) may be limited by external diffusion of reagents. The main stage (3–5 minutes) is limited by the diffusion of reagents (HF/H+) through the reaction product layer formed around Ta semi-product particles. The final stage (5–10 minutes) may be limited by the rate of the chemical reaction on the surface of unreacted cores or by diffusion within the porous layer of the product. The high conformity with the Jander model, valid for low degrees of conversion, is explained by the very rapid attainment of high α values in this process.
A methodology for the precipitation of potassium heptafluorotantalate (K2TaF7) from sulfate-fluoride solutions was developed. The obtained concentrate had a purity of 52.64 wt.% Ta compared to the theoretical 46.14 wt.% for pure K2TaF7.
The two-stage scheme developed (alkaline roasting + acid leaching with HF-H2SO4) demonstrated high efficiency in extracting tantalum from complex secondary raw materials, Ta-W semi-product from waste refractory alloy processing. Key advantages include:
− relative simplicity of equipment design;
− high tantalum extraction efficiency at the acid leaching stage (>99.99 wt.%);
− good selectivity concerning main impurities W and Mo during aqueous leaching;
− ability to operate at room temperature during the main dissolution stage of the Ta semi-product;
− use of widely available reagents (NaOH, H2SO4, HF).
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to this work as follows: the concept and research design, draft manuscript were developed, analysis and interpretation of results by I.D.T. and M.A.S.; data collection was carried out by M.A.S.; All authors reviewed the results and approved the final version of the manuscript.
AVAILABILITY OF DATA AND MATERIALS
The data is presented on the platform Zenodo, link: https://zenodo.org/records/17424564. X-ray diffraction pattern of the Ta-semi-product formed after leaching tungsten and molybdenum from the Ta-W intermediate product with alkalis.
ACKNOWLEDGEMENTS
Declared none.


