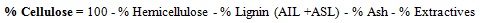RESEARCH ARTICLE
Chemical Kinetics of Alkaline Pretreatment of Napier Grass (Pennisetum purpureum) Prior Enzymatic Hydrolysis
Samuel Eshorame Sanni*, Olasubomi Akinrinola, Esther Ojima Yusuf, Omololu Oluwatobi Fagbiele, Oluranti Agboola
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 36
Last Page: 56
Publisher ID: TOCENGJ-12-36
DOI: 10.2174/1874123101812010036
Article History:
Received Date: 27/12/2017Revision Received Date: 31/01/2018
Acceptance Date: 8/04/2018
Electronic publication date: 30/04/2018
Collection year: 2018

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Napier grass is a naturally abundant waste material that can be cultivated over a vast area of land which makes it a viable source for sugar and bioethanol production.
Introduction:
The presence of lignin in the biomass makes cellulose inaccessible for conversion to useful products, however, in order to provide for efficient utilization of the waste material, reagent and energy, a study on the kinetics of lignin removal from Napier grass was carried out in this work using 1 and 3 w/w % NaOH at temperatures between 80 and 120°C.
Materials & Methods:
Based on the investigation, there was increased lignin removal for increased NaOH concentration. Kinetic parameters were also determined and it was observed that, the reaction of lignin in Napier grass with NaOH obeys a pseudo-zero or pseudo-fractional order kinetics. Furthermore, the orders of the reaction for the pretreatment conditions of 3 w/w% NaOH at 100°C and those of 3 and 1 w/w NaOH at 120°C gave close reaction orders of 0.2, 0.22 and 0.24 respectively after 110 minutes, which implies that, for the three cases, the residual lignin in the extract was almost the same at the pretreatment conditions while slight differences are evident in their pseudo rate constants. Also, it was observed that, the activation energy of the reaction reduced significantly as the concentration of NaOH increased from 1w/w - 3 w/w%.
Conclusion:
Based on the AIL and the total lignin (i.e. AIL + ASL) in the Napier grass, the recorded delignification efficiencies at the optimum pretreatment time of 17.5 h are 90 and 76% respectively. In addition, the adopted Differential Technique (DT) combined with the Ostwald Method of Isolation (OMI) can be accurately used to study the kinetics of lignin removal from Napier grass.
1. INTRODUCTION
Since the Vietnam war, energy availability has become increasingly critical [1]. Potential sources of fuel energy include solar, wind, geothermal, nuclear and other forms of renewable energy. First-generation biofuels are produced from food crops such as sugar cane and corn [2]. However, food competition led to the emergence of second-generation biofuels which are sourced from non-food feedstock and agricultural wastes while third-generation biofuels find their origin in synthetic fibres [3]. Fossil fuels used to be the most abundant energy sources but their continuous depletion have given rise to adverse environmental effects. According to recent works, waste materials are being exploited as very good sources of energy. Amongst the available waste materials, Napier grass (Pennisetum purpureum) was selected for this research because of its high productivity per hectare, low cost, its adaptability to infertile land and the usefulness of the whole plant [4]. Napier grass can be converted to sugar or ethanol. In the production of bioethanol from cellulose, the cellulose is first separated from lignin and hemicellulose by pretreatment methods. The purpose of the pretreatment is to provide easy enzyme-accessibility of carbohydrate polymers [5], remove lignin, reduce cellulose crystallinity, increase the porosity of the materials, improve substrate ability to form sugars and prevent loss of carbohydrate and the formation of byproducts which are inhibitory to hydrolysis and fermentation processes. Some bases/alkalis can also be used for pretreatment of lignocellulosic materials and the effect of alkaline pre-treatment depends on the lignin content of the materials [6]. Amongst the pretreatment technologies, alkaline pretreatment has received much attention because it is less energy intensive and relatively inexpensive [7]. After pretreatment, the substrate is then hydrolyzed using chemicals/enzymes. Enzymes are very active on cellulose because they both have a similar chemical structure. Using acid as substitute for enzymes is not appropriate for hydrolysis because of the high temperature requirement and the issue of acid corrosion, whereas the use of enzymes guarantee higher specificity and more neutral reaction without the formation of byproducts [4, 8]. However, pretreatment/delignification kinetics may be used to optimize the production of high value sugars or maximize utilization of feed stocks nutrients. Besides providing access route to the cellulose in biomass, initial pretreatment of biomass and enzymatic hydrolysis lead to the recovery of residual lignin which can be converted to p-hydroxy-phenyl propane by taking advantage of the structural adjustments in the lignin matrix on further pretreatment [8, 9]. In a similar work, it was asserted that depolymerization and condensation reactions are the mechanisms responsible for lignin removal during biomass pretreatment [10]. Based on literature, the mechanisms for lignin removal from plant biomass bring about size reduction of the substrate, physical redistribution of the components, depolymerization and solubilization [11]. Shea-tree sawdust delignification kinetics has been investigated [12]. The raw material was pretreated at 120-150°C. Based on their results, they concluded that the peroxide pretreatment adopted for lignin removal improved the biofuel yield.
In a recent study, the kinetics of lignin removal from corn Stover was carried out by soaking the biomass in different concentrations of aqueous ammonia aided by thermal treatment between 30 and 70°C [13]. Also, the pretreatment of corn Stover was conducted at temperatures of 170-230°C and 150-210°C using hot water and dilute acid to remove lignin from the substrate and it was discovered that pretreatment conditions from 200-230°C did not enhance lignin removal from the biomass [14]. The comparative kinetics of sodium-chlorite acetic acid and peracetic acid delignification of four biomass samples (switch grass, pine saw dust, poplar and corn Stover) was studied [15] with a record of >90% delignification efficiency, however, they added that, the choice of pretreatment for biomass delignification is substrate dependent; similar works were carried out using acid and alkali pretreatments for poplar and switch grass [16] and oat hull biomass [17]. It has been reported that delignification enhances sugar yields as high as over 90% for biomasses such as woods, grasses and corn [18]. They also discussed the advantages of several pretreatment technologies prior hydrolysis and fermentation. The structural modifications of lignin occurring in the stages of maturity of Napier grass have been discussed [19]. The physicochemical changes occurring in lignocellulosic biomasses during pretreatment can be determined experimentally, which will aid the development of models for accurate design of pretreatment processes [20]. Modelling and optimization of Napier grass pretreatment using four combined pretreatment techniques for glucose and xylose production was carried out [21], where they reported lower sugar yield from NaOH and moist heat, and NaOH and microwave hybridized pretreatment methods as compared to the HCl-hybridized forms of pretreatments; two reasons may have been responsible for the lower sugar yield and these include, the interference of thermal radiation with the action of NaOH in the sample and the selectivity of the lignin matrix for NaOH. Lignin binds to sites within a biomass/substrate especially at areas where it is least desired for industrial applications [22-27].
Therefore, the objective of this study is to investigate the alkaline pretreatment kinetics of lignin removal in Napier grass under different pretreatment conditions using the differential rate law and the method of isolation because previous works on Napier grass pretreatment have not exploited the application of these methods in determining the kinetic parameters. Also, first-order kinetics is usually assumed for wood as biomass substrate which may not necessarily suffice for other biomasses.
2. MATERIALS, REAGENTS AND EQUIPMENT
40-day old Napier grass (Pennisetum purpureum) was harvested from Covenant University surrounding, in Ota, Ogun state. It was separated from impurities (plants, leaves, sand, stones and dirt). The separated Napier grass was washed and air dried for 7 days. It was then milled and sieved using a 500 micron-sieve. After which the milled grass was stored in sealed plastic bags at room temperature for characterization and pretreatment. The following reagents were used for pretreatment and hydrolysis: NaOH of 99.8% made by Fischer chemical, H2SO4 of 97% purity made by Sigma Aldrich, Absolute ethanol of 99.9% purity-analytical grade by Fischer chemical, CaCO3 of 99% purity by Nikitia Minchem Industries, Distilled water, Sodium citrate buffer (pH 4.8), Dinitrosalisilic acid reagent (DNSA reagent).
UV mass spectrophotometer: 7310 visible Scanning Spectrophotometer fitted with 10 x 10 mm cuvette holder, 320 - 1000nm wavelength, resolution = 1nm accuracy of ±nm, manufactured by Jenway, Oven Vs-1202d3 by Vision Scientific Ltd., electronic weighing balance manufactured by Swastic Systems & Services, India, measuring cylinder, flat bottom flasks, conical flask and beakers manufactured by J Sill Borosilicates, filter paper by Whatman No. 2V and of 18 cm diameter.
3. EXPERIMENTAL PROCEDURE
3.1. Characterization of Napier grass (Pennisetum purpureum)
Napier grass (Pennisetum purpureum) was characterized prior and after alkaline pretreatment to determine the total percentage of cellulose, hemicellulose, lignin, extractives, moisture and ash in the biomass. Structural carbohydrates and lignin in the grass were determined based on National Renewable Energy Laboratory (NREL) procedures [14, 28]. The percent extractives, moisture and ash were determined based on the American Society of Testing and Material standards as used by Dussadee et al. [29].
3.1.1. Determination of Moisture Content
5 g Napier grass was weighed and put on cellulose paper of known weight. This was done with the aid of the weighing balance. The sample was placed in a crucible and transferred to an oven at 105°C. After 1 h, the sample was allowed to cool in the desiccator for 15 mins. The weight of the sample was taken and recorded. The sample was further dried by putting it in the oven for another 3 h at 30 minutes interval at 105°C until a constant weight was achieved. The samples were removed and allowed to cool in the desiccator for 15 mins. The total dry solids weight and the %moisture of the sample were determined using equations (1) and (2) respectively.
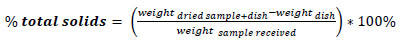 |
(1) |
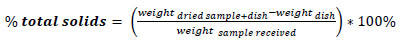 |
(2) |
3.1.2. Determination of Extractives
5 g of Napier grass biomass was weighed and poured onto a cellulose paper of known weight which was folded to take the shape of the thimble of the Soxhlet extractor. The paper and its content were then placed in the thimble of the Soxhlet extractor. With the aid of a volumetric flask, 190 ml of ethanol was measured and transferred into the round bottom flask.
The Soxhlet extractor was then mounted on the heating mantle for 8 hours for extraction to take place. After extraction, the sample was air dried for 24 hours and put in the oven set at 105°C until constant weight was achieved; the ordinary dry weight of the sample was then determined using equation (3). The solvent containing the extractives was now heated to evaporate, in order to leave the extractives as residue. The weight of the round bottom flask plus the extractives was then determined and recorded. The %extractives was then obtained from equation (4).
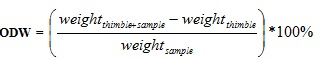 |
(3) |
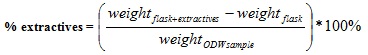 |
(4) |
3.1.3. Estimation of %hemicellulose
A standard solution of 0.5 M NaOH was produced with distilled water. 1 g of the extractive free Napier grass sample was weighed and kept inside a 250 ml Erlenmeyer flask. With the aid of volumetric flask, 150 ml of NaOH was weighed and poured into the flask containing 1 g of the sample. The Erlenmeyer flask was placed inside the oven whose temperature was adjusted to 100°C. The sample was then allowed to boil for 4 h. After heating and the reaction time elapsed, the sample was brought out of the oven and allowed to cool to room temperature. Then the sample was filtered via Buchner funnel vacuum filtration set up and a pump; cellulose filter paper was also used to carry out the filteration. The sample residue was then washed with distilled water until the pH of the filterate was 7 in order to ensure that all the NaOH in the sample had been removed. The sample was dried in the oven at temperature of 105°C to a constant weight. Thereafter, the sample was then allowed to cool in the dessicator with the dry weight of the sample measured using a weighing balance. The Hemicellulose content of the Napier grass was then obtained using equation (5).
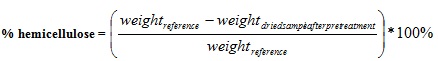 |
(5) |
3.1.4. Determination of Lignin and Ash Content Procedure
Two types of lignin were accounted for:
3.1.4.1. Insoluble Lignin
300 mg of extractive free sample was weighed and put inside a test tube. 3 ml of 72 w/w % H2SO4 was measured and added to the sample for complete hydrolysis of the cellulose and hemicellulose. The test tube was swirled gently to allow for complete mixing and access of the sulfuric acid to the whole material. The sample was then kept at room temperature for 2 h while shaking at 30 mins interval to allow for complete hydrolysis. The sample was transferred carefully to a 250 ml Erlenmeyer flask and 84 ml of distilled water was added. The sample was autoclaved for 1 h at 121°C and then cooled to room temperature. The sample was filtered with the aid of the filtering crucible and filter paper. Then the residue was dried at 105°C in an oven till constant weight then the insoluble residue weight was recorded and using equation (3), the Ordinary Dry Weight (ODW) of the Sample was calculated. The residue was poured into a crucible and the total weight of crucible and sample was recorded. The crucible + sample was then put in the furnace for 6 h at 575°C to obtain the ash content. The ash content was subtracted from the total amount of insoluble residue to obtain the quantity of insoluble lignin using equation (6).
3.1.4.2. Soluble Lignin
3 ml of the filtrate obtained in section 3.1.4.1 was taken to the UV-Spectrometer to determine the absorbance of the sample at a wavelength of 240 nm. The Acid Insoluble and Acid Soluble Lignin were obtained from equations (7) and (8) respectively.
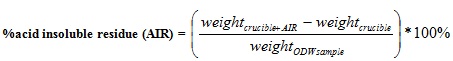 |
(6) |
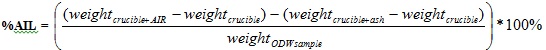 |
(7) |
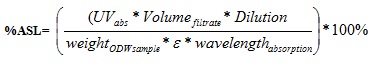 |
(8) |
The modified Beer Lambert’s equation:
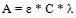 |
(9) |
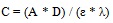 |
(10) |
Where:
A = absorbance at 240 nm
D = dilution factor
λ = absorption wavelength
ε = absorptivity and C = lignin concentration in NaOH
3.2. Alkaline Pretreatment of Napier Grass (Pennisetum purpureum)
1 g of Napier grass was treated in a 250 mL Erlenmeyer flask with 1 and 3 w/v separate solutions of 50 mL NaOH between 80-120°C at a temperature interval of 10°C and time interval of 30 minutes for a total of 45 runs using Table 1. The samples were withdrawn, filtered and analyzed using a UV spectrophotometer. The flask was covered with aluminum foil and placed in the oven. Alkaline method of pretreatment was selected because alkaline pretreatment can take place at temperatures as low as 45-50°C and pressure of 1 atm compared to other pretreatment methods [30]. Also, in terms of reactivity, alkaline pretreatment is safer relative to acid pretreatment because of the tendency of side reactions. Alkalis are relatively cheap and can be recovered by carbonating with CO2 [31].
| t, min |
Abs. at 80°C |
Conc. at 80°C |
Abs. at 90°C |
Conc. at 90°C |
Abs. at 100°C |
Conc. at 100°C |
Abs. at 110°C |
Conc. at 110°C |
Abs. at 120°C |
Conc. at 120°C |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0.14 | 0.28 | 0.24 | 0.49 | 0.30 | 0.61 | 0.41 | 0.81 | 0.75 | 1.50 |
| 60 | 0.26 | 0.53 | 0.55 | 1.09 | 0.73 | 1.46 | 1.10 | 2.2 | 1.49 | 2.99 |
| 90 | 0.56 | 1.12 | 0.97 | 1.94 | 1.32 | 2.64 | 2.44 | 4.88 | 2.53 | 5.07 |
3.3. Determination of kinetic parameters
3.3.1. Determination of Reaction Orders with respect to NaOH
Given the reaction between Lignin (A) and NaOH (B) to give product (C) is represented by the following stoichiometric relationship as given in equation (12):
 |
(12) |
Rate of disappearance of A is given as:
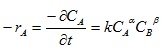 |
(13) |
Assuming NaOH is in excess relative to the volume of A (lignin), the method of isolation then applies thus;
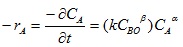 |
(14) |
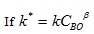 |
(15) |
Therefore, from
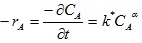 |
(16) |
Linearizing equation (16) gives:
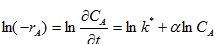 |
(17) |
Where:
-rA = dCA/dt = the rate of disappearance of lignin from Napier grass
CA = concentration of residual lignin in the biomass
k* = pseudo rate constant or pseudo velocity constant for the reaction
k = true rate constant or true velocity constant for the reaction
α = the order of the reaction with respect to lignin
β = the order of the reaction with respect to NaOH
From equation (17), a plot of ln (-rA) vs ln CA gives α as the slope and ln k* as intercept
In order to obtain the true rate constant of the reaction, the order of reaction with respect to NaOH (B) and the overall order of the reaction, several initial concentrations of NaOH can be used by repeating the experiments in section 3.2 having determined the pseudo rate constant and order of the reaction with respect to lignin. The varied initial concentrations of NaOH will then be measured experimentally alongside their corresponding pseudo rate constants. Afterwards, the natural log of equation (15) is taken which informs that, a plot of the pseudo rate constant against the initial NaOH concentration will give β as the reaction order with respect to NaOH with ln k as the intercept. In addition, the natural log inverse of the intercept can then be obtained which gives the value of the true rate constant for the reaction.
However, since the true rate constants variation are difficult to determine for varied concentrations of NaOH, the pseudo rate constants for the reaction were used to estimate the activation energies for the reactions at the different conditions.
3.3.2. Determination of Activation Energy for the Reaction between NaOH and Lignin in Napier Grass
From the Arrhenius Equation:
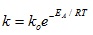 |
(18) |
Where:
k = rate constant at a specified temperature (used as pseudo rate constant)
ko = the pre-exponential factor
EA = activation energy of the reaction between NaOH and lignin
T = the reaction temperature
e = inverse of natural log
R = gas constant (8.314 J/mol.K)
Taking the natural log of equation (18) gives:
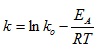 |
(19) |
3.3.3. Determination of Optimum Pretreatment Time
For the pretreatment temperatures considered, the time of pretreatment was extended for the pretreatment temperature that gave the highest amount of lignin in the extract/NaOH. This was done in order to conserve energy and resources with a view of staying within the best pretreatment conditions that are mostly recommended for biomass pretreatment. The results obtained by varying pretreatment time and obtaining the corresponding absorbance alongside the concentrations of lignin in the extract of the sample at 240 nm is as shown in Table 2.
4. RESULTS AND DISCUSSION
The results from Napier grass compositional analysis is given in Fig. (1).
4.1. Compositional Analysis
Considering Fig. (1), the Napier grass consists mainly of cellulose, hemicellulose, Acid Insoluble Lignin (AIL), extractives, moisture, ash, Acid Soluble Lignin (ASL).
 |
Fig. (1). Percent Composition of the Napier grass. |
4.2. Calibration Graph
The concentration of lignin in NaOH after each pretreatment condition was determined by UV-visible spectrophotometer which was used to read the absorbance at a wavelength of 240 nm and then the modified Beer-Lambarts equation was used to calculate the lignin concentration with a molar absorptivity coefficient value of 25 L(gcm)-1.
4.2.1. Effect of Temperature and Time of Pretreatment
At increased temperatures and pretreatment time, for 1 w/w% NaOH, the absorbance increased, which is an indication of higher lignin solubility and removal from Napier grass (Table 1 and Fig. 2). Maintaining the same concentration of NaOH at 120°C gave the highest quantity of adsorbed lignin. Therefore, for effective pretreatment of 1 g Napier grass sample using 1 w/w% NaOH, the best operating conditions within the limits of the pretreatment conditions applied, is heating the sample at 120°C for 90 mins.
 |
Fig. (2). Standard glucose concentration vs. absorbance. |
4.2.2. Delignification Kinetics
The kinetic data was generated for a pretreatment time of 100 mins.
4.2.3. Delignification Kinetics of 1 w/w% NaOH
The graphical illustration of lignin concentration variation with time is as shown in Fig. (3). The plot shows that the concentration-time graph is non-linear. Between 80-120°C, the concentration of lignin dissolved in NaOH increased between 0 and 100 mins. When the pretreatment temperature was raised by 10°C, the quantity of lignin removed (lignin in NaOH) after pretreatment was 2.25 mg/mL at 90°C as compared to 1.4 mg/L at 80°C, for the same pretreatment time (100 mins). From the plot, the change in concentration versus time was established in order to determine the rate of lignin removal from the sample (Table 3). Afterwards, a plot of natural log of rate versus natural log of concentration was made (Tables 4 and 5) by assuming that the reaction mixture of NaOH and Napier grass is homogenous so that the reactants and products are considered to be in the same phase before filtration.
| Time, mins | Absorbance, - | Concentration mg/mL |
|---|---|---|
| 0 | 0 | 0 |
| 30 | 0.889 | 1.778 |
| 60 | 1.689 | 3.378 |
| 90 | 2.884 | 5.768 |
| 120 | 3.442 | 7.351 |
| 150 | 4.215 | 9.544 |
| 180 | 5.611 | 13.512 |
| 210 | 6.540 | 16.15 |
| 240 | 7.913 | 20.041 |
| 270 | 8.770 | 22.483 |
| 300 | 9.782 | 26.48 |
| 330 | 10.55 | 29.51 |
| 360 | 11.68 | 33.95 |
| 390 | 12.671 | 37.9 |
| 420 | 13.961 | 43.053 |
| 450 | 14.882 | 45.46 |
| 480 | 15.776 | 47.81 |
| 510 | 16.661 | 50.15 |
| 540 | 17.481 | 52.33 |
| 570 | 18.983 | 56.32 |
| 600 | 19.59 | 57.8 |
| 630 | 20.896 | 60.96 |
| 660 | 21.627 | 61.84 |
| 690 | 23.145 | 63.61 |
| 720 | 24.973 | 65.77 |
| 750 | 26.499 | 67.57 |
| 780 | 29.015 | 70.14 |
| 810 | 31.76 | 72.67 |
| 840 | 34.785 | 75.50 |
| 870 | 38.11 | 78.59 |
| 900 | 41.978 | 80.23 |
| 930 | 46.501 | 82.17 |
| 960 | 48.677 | 83.15 |
| 990 | 52.98 | 85.07 |
| 1020 | 52.979 | 85.07 |
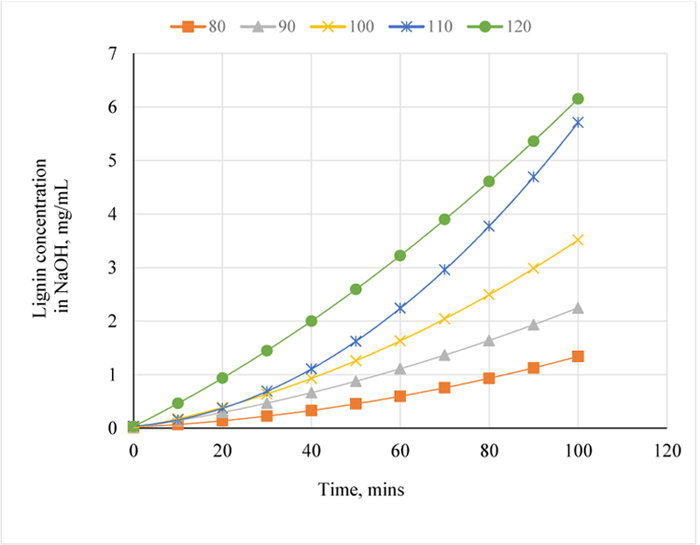 |
Fig. (3). Lignin Concentration in filtrate with time for 1 wt/wt % NaOH Pretreatment. |
| Time, mins |
ΔC/Δt mg/mL.min at 80°C |
ΔC/Δt mg/mL.min at 90°C |
ΔC/Δt mg/mL.min at 100°C |
ΔC/Δt mg/mL.min at 110°C |
ΔC/Δt mg/mL.min at 120°C |
|---|---|---|---|---|---|
| 0 | - | - | - | - | - |
| 10 | 0.0051 | 0.0154 | 0.0171 | 0.0117 | 0.0432 |
| 20 | 0.0069 | 0.0174 | 0.0211 | 0.0217 | 0.0472 |
| 30 | 0.0087 | 0.0194 | 0.0251 | 0.0317 | 0.0512 |
| 40 | 0.0105 | 0.0214 | 0.0291 | 0.0417 | 0.0552 |
| 50 | 0.0123 | 0.0234 | 0.0331 | 0.0517 | 0.0592 |
| 60 | 0.0141 | 0.0254 | 0.0371 | 0.0617 | 0.0632 |
| 70 | 0.0159 | 0.0274 | 0.0411 | 0.0717 | 0.0672 |
| 80 | 0.0177 | 0.0294 | 0.0451 | 0.0817 | 0.0712 |
| 90 | 0.0195 | 0.0314 | 0.0491 | 0.0917 | 0.0752 |
| 100 | 0.0213 | 0.02246 | 0.0531 | 0.1017 | 0.0792 |
| Time, mins |
lnCc mg/mL at 80°C |
lnCc mg/mL at 90°C |
lnCc mg/mL at 100°C |
lnCc mg/mL at 110°C |
lnCc mg/mL at 120°C |
|---|---|---|---|---|---|
| 0 | -3.98459 | -5.116 | -5.59942 | -3.30771 | -3.48349 |
| 10 | -2.66499 | -1.96611 | -1.74469 | -1.8734 | -0.77068 |
| 20 | -1.97616 | -1.22418 | -0.9527 | -0.99263 | -0.06753 |
| 30 | -1.48899 | -0.75929 | -0.45146 | -0.37455 | 0.369285 |
| 40 | -1.1069 | -0.41249 | -0.07505 | 0.099483 | 0.692497 |
| 50 | -0.79054 | -0.13239 | 0.230079 | 0.483413 | 0.951928 |
| 60 | -0.51987 | 0.10436 | 0.488396 | 0.805851 | 1.17022 |
| 70 | -0.28289 | 0.310422 | 0.713293 | 1.083702 | 1.359617 |
| 80 | -0.07913 | 0.493476 | 0.912965 | 1.327764 | 1.527512 |
| 90 | 0.118316 | 0.658556 | 1.092829 | 1.545347 | 1.678721 |
| 100 | 0.291624 | 0.809151 | 1.25667 | 1.741623 | 1.816566 |
| Time |
Absor- bance |
Concen- tration (mg/mL) |
Absor- bance |
Concen- tration (mg/mL) |
Absorb- ance |
Concen- tration (mg/mL) |
Absorb- ance |
Concen- tration (mg/mL) |
Absorb- Ance |
Concen- Tration (mg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| (mins) | (80°C) | (80°C) | (90°C) | (90°C) | (100°C) | (100°C) | (110°C) | (110°C) | (120°C) | (120°C) |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0.216 | 0.432 | 0.307 | 0.61 | 0.413 | 0.826 | 0.505 | 1.01 | 0.889 | 1.778 |
| 60 | 0.361 | 0.722 | 0.63 | 1.26 | 0.859 | 1.718 | 1.223 | 2.446 | 1.689 | 3.378 |
| 90 | 0.779 | 1.558 | 1.105 | 2.21 | 1.438 | 2.876 | 2.541 | 5.082 | 2.884 | 5.768 |
4.2.4. Theoretical Estimation of the Kinetic Parameters
Fig. (4) gives an illustration of the variation of residual lignin concentration in the filtrate with the rate of lignin removal for the pretreatment process at 80°C. Going by equation (16), the order of the reaction is 0.50 (fractional order); the intercept simply gives ln k* = -3.9979 hence, k* = ln-1 (-3.9979) = 0.018 mg0.5/mL0.5.min is the rate constant for lignin removal from the Napier grass sample i.e. the point on the ordinate where the concentration of lignin = 0.
 |
Fig. (4). ln (ΔCc/Δt) vs. ln (Cc) for 1 w/w% NaOH pretreatment of Napier grass at 80°C. |
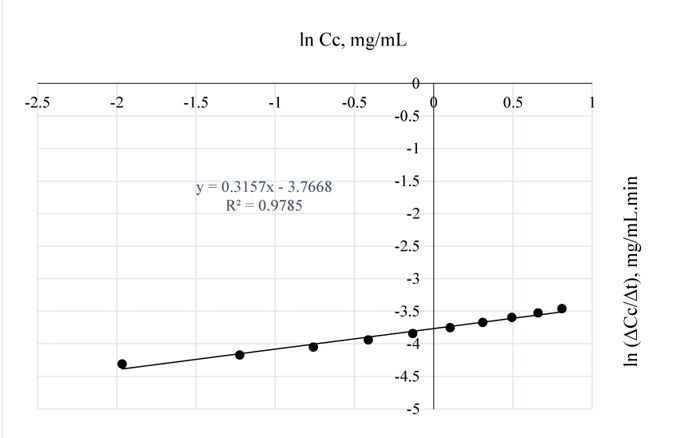 |
Fig. (5). ln (ΔCc/Δt) vs. ln (Cc) for 90°C pretreatment. |
Again, comparing Figs. (4 and 5), the order of the reaction decreased from 0.5 to 0.3 when the temperature of the pretreatment process was increased by 10°C hence, the positive difference in temperature favoured higher lignin removal. Also, the lower reaction order obtained here as compared to the order at 80°C, shows that more lignin was removed at 90°C since rate of reaction is a measure of the residual lignin concentration in Napier grass. The intercept simply gives ln k* = -3.7668 hence, k* = ln-1 (-3.2722) = 0.023 mg0.7/(mL)0.7min is the rate constant for the lignin and NaOH reaction for which the effect of 1 wt/wt % NaOH had no effect on the sample, because, at that point on the horizontal axis, the concentration of lignin in NaOH = 0 i.e. at that concentration, there is no feasible reaction between lignin in the substrate and the alkali. Figure (5) gives the concentration of lignin in the alkali at 90°C.
The concentration-time relationship in Fig. (3) shows that, at 100°C, the amount of lignin removed was 3.51 mg/mL NaoH after 100 mins of pretreatment. However, from Fig. (6), the order of reaction for NaOH and lignin in Napier grass is 0.4 with R2 value of 0.99. Comparing this result with the previous values (i.e. reaction orders) obtained at 80 and 90°C, the reaction order at 100°C is indicative of higher residual lignin in the Napier grass at 100 than at 90°C but lower than that at 80°C.
This could have resulted from the dissolution of other components such as extractives extracted alongside the lignin by the NaOH which may have posed some form of resistance to the reaction of NaOH and lignin. Here, the reaction pseudo rate constant is 0.031 mg0.6/L0.6.min. Also, the rate of reaction of lignin and NaOH began to decrease after 90 mins of extraction time which may be undesirous, although, reaction rate is expected to decrease as a reaction approaches completion.
In Fig. (3), at 110°C, the amount of lignin removed per mL of NaOH was 5.71 mg which is 62.7% higher than the amount of lignin removed at 100°C and considering Fig. (7) and Tables 3-4; see also the values obtained at other temperatures, the order of the reaction increased to 0.6 for the conditions at 100°C. In Fig. (6), the rate of lignin removal from the biomass can be determined at any specified concentration of lignin in NaOH solution by first obtaining the natural log of that concentration value and tracing the corresponding value on the ordinate whose ln-1 or exponent is then obtained as the rate value. The value of the pseudo rate constant = 0.038 mg0.4/mL0.4min.
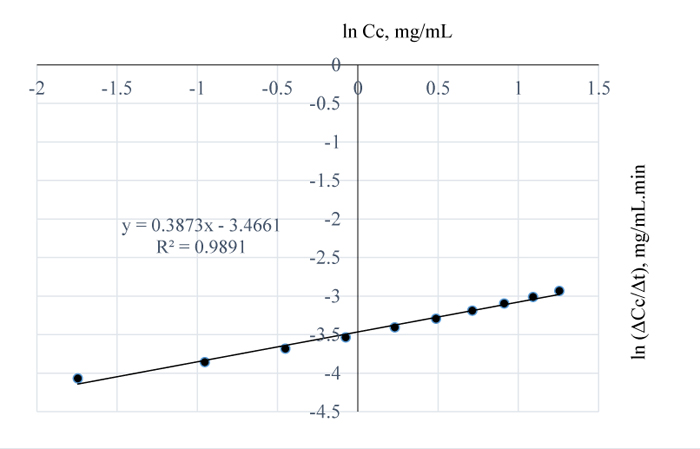 |
Fig. (6). ln (ΔCc/Δt) vs. ln (Cc) for 100°C pretreatment. |
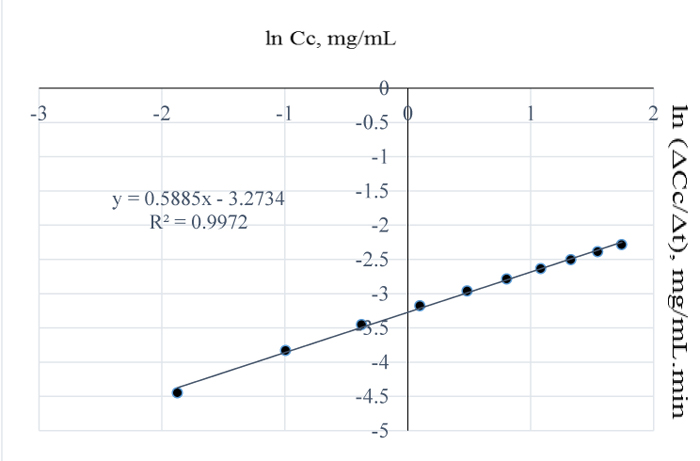 |
Fig. (7). ln (ΔCc/Δt) vs. ln (Cc) for 110°C pretreatment. |
At 120°C, the quantity of lignin removed per mL NaOH is 6.15 mg (Fig. 3) which is 7.7% higher than the amount of lignin removed at 110°C. From the plot of natural logs of rate and concentration, it is evident that the rate of lignin removal is temperature dependent, which implies that, an increase in temperature has some/little effect on the rate of reaction; the order of the reaction is 0.2. This is further justified by the lowest slope obtained at this temperature which also implies that, the residual lignin concentration in the substrate is very low hence, high disappearance of lignin from Napier grass (Fig. 8). Furthermore, the % extractives have no resistance to the reaction as they may have been vapourized at that temperature. Here, the value of the pseudo rate constant is 0.049 mg0.8/mL0.8min.
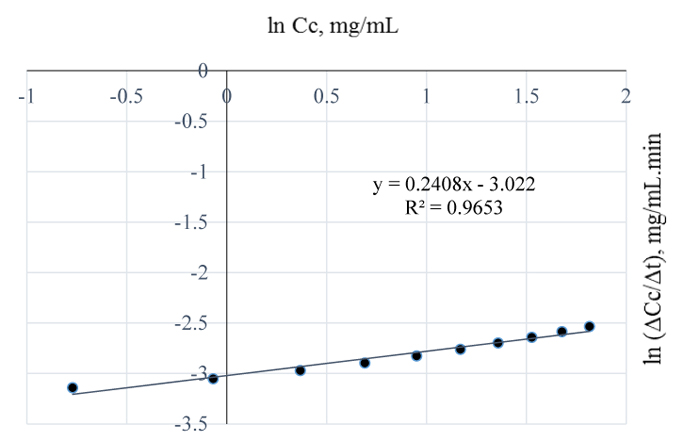 |
Fig. (8). ln (ΔCc/Δt) vs. ln (Cc) for 120°C pretreatment of Napier grass with 1 w/w% NaOH. |
4.3. Delignification Kinetics of 3 w/w% NaOH
Table 5 gives the absorbance of the NaOH filtrate when it was placed on the UV-Vis spectrophotometer. The absorbance of the NaOH filtrate increased with time and temperature i.e. from 0-90 mins and between 80-120°C.
 |
Fig. (9). Lignin Concentration in filtrate with time for 3 wt/wt % NaOH Pretreatment. |
As seen for the same pretreatment time and temperature for 1 w/w % NaOH pretreatment, the concentration of lignin in solution increased with time and temperature for 3 w/w% NaOH pretreatment; this is supported by the results obtained by Wongwatanapaiboon et al. [13], although, in their work, ammonia was used to pretreat Napier grass. Here, the highest quantity of lignin removed from the Napier grass sample was recorded at 120°C and after 90 mins Fig. (9).
Figs. (10 and 11) are plots of the natural logs of concentration and time for 3 w/w% NaOH pretreatment of Napier grass at 80 and 90 °C respectively. From the plots, the rate of removal of lignin from Napier grsass is not dependent on concentration since the reaction order is 1 for both cases. However, between 80 and 90°C, lignin removal was temperature dependent as a 10°C rise in temperature increased the quantity of lignin removed from Napier grass with the reaction rate constants at both conditions estimated as 0 mg/mL.min.
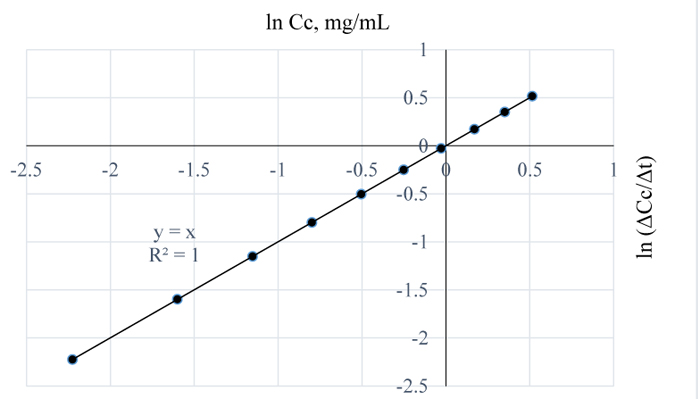 |
Fig. (10). ln (Δc/Δt) vs. time for 3 w/w% NaOH pretreatment of Napier grass at 80°C. |
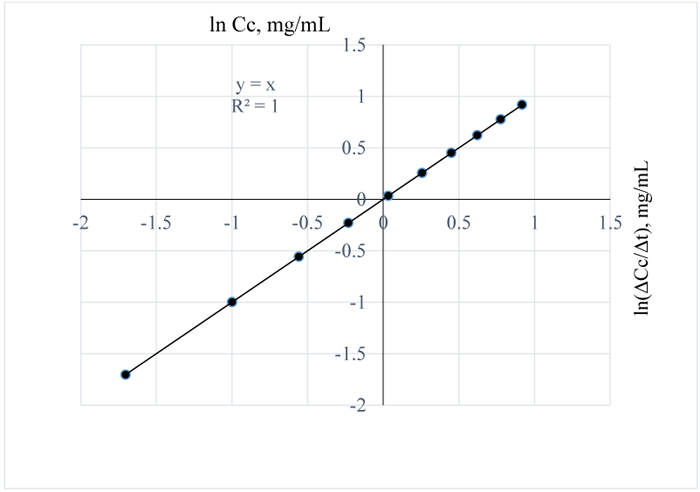 |
Fig. (11). ln (Δc/Δt) vs. time for 3 w/w% NaOH pretreatment of Napier grass at 90°C. |
In Fig. (12), the residual lignin concentration is low at 100°C for 3 w/w% NaOH pretreatment of the sample; the order and pseudo rate constants for this pretreatment condition are 0.2 and 0.03 mg0.8/mL0.8min respectively; a similar reaction order was obtained for the extraction at 120°C and for 1 w/w% NaOH. Also, the pretreatment of Napier grass using 1 w/w % NaOH at 80°C and 3 w/w% at 110°C, are indicative of similar removal rate of lignin from the sample. For the pretreatment conditions of 3 w/w % NaOH, the reaction orders with their corresponding pseudo rate constants as determined from Figs. (13 and 14) are 0.5, 0.045 mg0.5/mL0.5min and 0.2, 0.053 mg0.8/mL0.8min respectively.
 |
Fig. (12). ln (Δc/Δt) vs. time for 3 w/w% NaOH pretreatment of Napier grass at 100°C. |
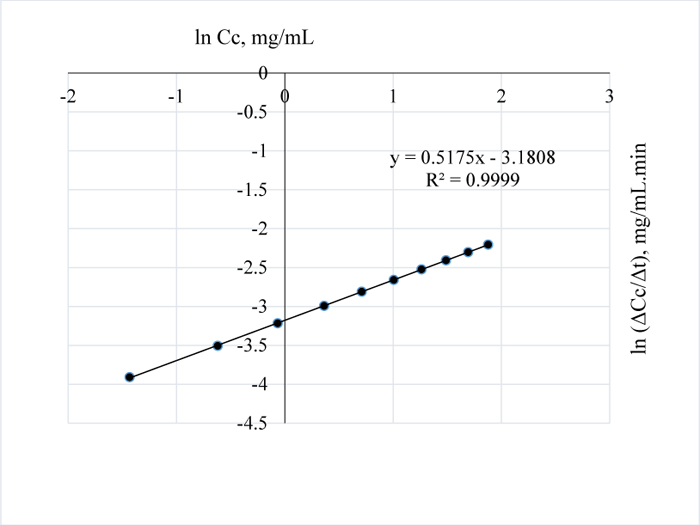 |
Fig. (13). ln (Δc/Δt) vs. time for 3 w/w% NaOH pretreatment of Napier grass at 110°C. |
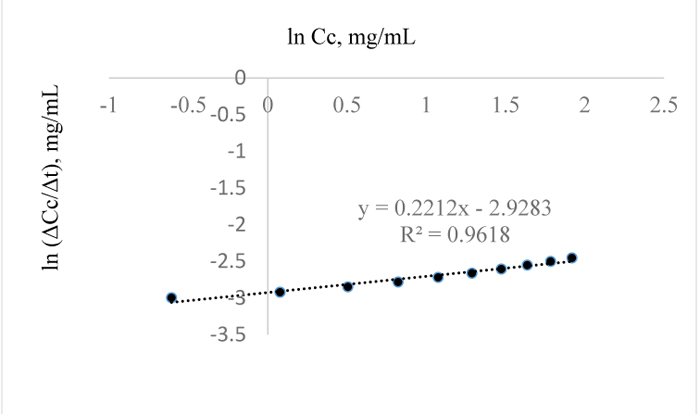 |
Fig. (14). ln (Δc/Δt) vs. time for 3 w/w% NaOH pretreatment of Napier grass at 120°C. |
| Temp.°C |
Initial lignin conc. for 1 w/w% |
Final lignin conc. for 1 w/w% |
Initial lignin conc. for 3 w/w% |
Final lignin conc. 3 w/w% |
Δ 1 w/w% Lignin Conc. |
Δ 3 w/w% Lignin Conc. |
% difference (Δ3-Δ1)/Δ1 * 100% |
|---|---|---|---|---|---|---|---|
| 80 | 0.019 | 1.339 | 0.034 | 1.674 | 1.32 | 1.64 | 24 |
| 90 | 0.006 | 2.246 | 0.014 | 2.504 | 2.24 | 2.49 | 11 |
| 100 | 0.0037 | 3.5137 | 0.01 | 3.25 | 3.51 | 3.24 | 8 |
| 110 | 0.0366 | 5.7066 | 0.039 | 6.5387 | 5.67 | 6.4997 | 15 |
| 120 | 0.0307 | 6.1507 | 0.048 | 6.8184 | 6.12 | 6.7704 | 10 |
Considering Table 6, for all pretreatment conditions, if a benchmark is pegged at the conditions for 100°C, based on the %difference (8, 15 and 10% at 100, 110 and 120°C respectively) obtained for the amount of lignin extracted for the pretreatment conditions of 100-120°C for 1 and 3 w/w% NaOH, the rate of lignin removal is more temperature dependent than for conditions between 80 and 100°C. Also, the rate of lignin removal is more concentration dependent between 80-100°C than for conditions at 100-120°C due to the higher %difference (i.e. 24, 11 and 8% for 80, 90 and 100°C respectively) in concentration of lignin recorded, which is corroborated by the findings in [31]. See Table 6 for a summary of the pretreatment results between 80-120°C.
Considering the initial and final pretreatment conditions for the Napier grass sample, the results in Table 6 further justify the best pretreatment conditions as already mentioned and are in agreement with the findings of Mafuleka and Kanal [21] at increased pretreatment temperature. However, there seems to be some peculiarity in the results obtained i.e. the results clearly indicate that, the pretreatment conditions for 3 w/w % NaOH at 100°C and 120°C gave the least % difference of 8 and 10% respectively, which confirms the level of similarity between both pretreatment conditions hence, the established rate law at the condition for the highest lignin extraction is given as: –rA = k*CA0.2
Furthermore, the results from pretreatment at 120°C for 3 w/w% concentration of NaOH, reveal that the efficiency of lignin removal from the Napier grass sample was 72.2% based on the Acid Insoluble Lignin (AIL) content of the biomass but considering the total amount of lignin in the material, the delignification efficiency is about 59.2% for a pretreatment time of 90 minutes.
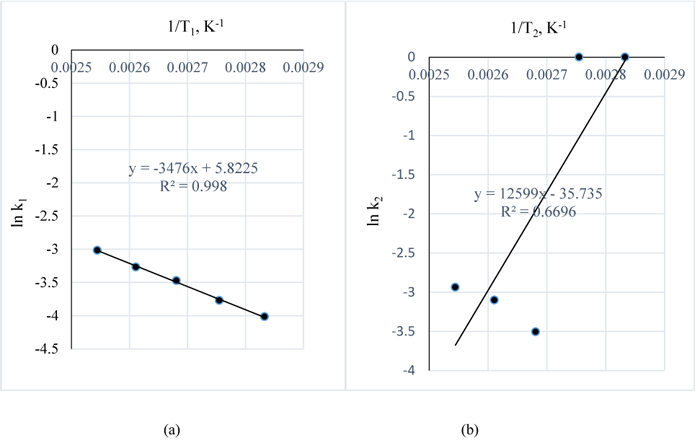 |
Fig. (15). a. ln k 1vs. 1/T1; b. ln k 2vs. 1/T2 |
The estimated k pseudo rate constants for the reaction are as given in Table 7. Using Equation (9), A plot of ln k for 1 w/w% NaOH vs. 1/T gives –EA/R as slope and ln ko as intercept while, a plot of ln k for 3 w/w% NaOH vs. 1/T gives –EA/R and ln ko as intercept.
| T, °C | k* for 1 w/w % pretreatment | k* for 3 w/w % pretreatment |
|---|---|---|
| 80 | 0.018 | 0 |
| 90 | 0.023 | 0 |
| 100 | 0.031 | 0.03 |
| 110 | 0.038 | 0.045 |
| 120 | 0.049 | 0.053 |
In Figs. (15a and b), –EA/R = -3476 and 12599 with R2 values of 0.99 and 0.67 giving EA values of 143404.9 and -519780.7 J/g.K, respectively. 1 g of lignin to mole = 1/343 gmol-1 (molar mass of lignin) = 0.002915 mole.
Since 0.002915 mole of lignin = 1 g of lignin and,
1 mole of lignin = 1/0.002915 = 343 g of lignin
:. R = 8.314 J/mol.K = 8.314 J/343g.K = 0.024239 J/g.K
Hence EA = 3476/0.024239 = 143404.9 J/g.K and -12599/0.024239 = -519780.7 J/g.K
This then reveals that the reaction is endothermic for the reaction conditions of 1 w/w % NaOH pretreatment and exothermic for the pretreatment conditions of 3 w/w % NaOH which is indicative of the ability of the reactants to generate the heat required for the process hence, there was excess heat during the reaction of 3 w/w % NaOH and Lignin. However, it is therefore recommended to carry out further research to investigate this ability of NaOH to react with lignin in Napier grass without any additional heat for 3 w/w% NaOH and higher concentrations.
Considering the results shown in Tables 1-8, an efficiency of 72% suggests the need for: a hybridized NaOH or combined pretreatment technique, longer pretreatment time or pretreatment at higher temperatures if a higher efficiency is desired. However, in this work, the pretreatment time was extended for the condition that gave the highest lignin removal. The results for time extension of the delignification process is as contained in Table 2; the variations of concentration of lignin in NaOH with the corresponding absorbance as obtaineded on the UV mass spectrometer were also recorded.
| Time, mins |
ln(ΔC/Δt) mg/mLmin at 80°C |
ln(ΔC/Δt) mg/mLmin at 90°C |
ln(ΔC/Δt) mg/mLmin at 100°C |
ln(ΔC/Δt) mg/mLmin at 110°C |
lnΔC/Δt) mg/mLmin at 120°C |
|---|---|---|---|---|---|
| 0 | - | - | - | - | - |
| 10 | -5.27851 | -4.17339 | -4.06868 | -4.44817 | -3.142 |
| 20 | -4.97623 | -4.05129 | -3.85848 | -3.83044 | -3.05336 |
| 30 | -4.74443 | -3.94248 | -3.68489 | -3.45144 | -2.97202 |
| 40 | -4.55638 | -3.84436 | -3.53702 | -3.17725 | -2.89679 |
| 50 | -4.39816 | -3.75502 | -3.40822 | -2.9623 | -2.82683 |
| 60 | -4.26158 | -3.67301 | -3.29414 | -2.78547 | -2.76145 |
| 70 | -4.14144 | -3.59721 | -3.19175 | -2.63526 | -2.70008 |
| 80 | -4.03419 | -3.52676 | -3.09887 | -2.5047 | -2.64226 |
| 90 | -3.93734 | -3.46095 | -3.0139 | -2.38923 | -2.5876 |
| 100 | -3.84905 | -3.79602 | -2.93558 | -2.28573 | -2.53578 |
4.4. Optimum Pretreatment Time for 3 w/w% NaOH Pretreatment of the Napier Grass
For all the pretreatment conditions considered, i.e. between 80-120°C, the 3 w/w% NaOH pretreatment of the Napier grass at 120°C gave the highest amount of lignin removed after 100 mins (Table 5) hence, in order to establish the best/optimum pretreatment time for the process, the pretreatment time was extended at a constant time interval of 30 minutes and it was observed that, for an optimum absorbance of 52.98, an optimum pretreatment time of 17.5 h is required to completely remove the AIL from the Napier grass (Table 2); the reports in [32] also confirms that higher absorbance values can be obtained for t-butyl-phenol or t-butyl phenoxide in an alkaline medium such as methanol at a wavelength of 203 nm. Also, the reaction order for the pretreatment process is closely related to a pseudo-zero order kinetics as shown in Fig. (15). The plot in Fig. (15) was made by first making a plot of Cc vs. t.
The appropriate polynomial equation was then displayed; this approach is valid and adopted due to the close data points generated from the progressive search for a steady state condition. Afterwards, the equation of the polynomial was differentiated to give the change in concentration wrt to time and because there are several close data points, few data points at a time interval of 120 minutes were selected and the plot of ln (∆Cc/∆t) vs. lnCc was then obtained with the final equation displayed within some limits of accuracy i.e. with R2 value of 0.86.
In Fig. (1), it is evident that the initial amount of lignin in 1.0 g of Napier grass is 0.0945 g but as the reaction approached completion Table (8), only 0.085 g was accounted for which shows that an efficiency of 90% was obtained for the removal of AIL with only 10% error which may have resulted from over saturation of the NaOH with lignin or inability of the NaOH to come in contact with the lignin as a result of the barrier to further dissolution of the lignin in NaOH. Furthermore, it is possible that the unreacted AIL and ASL were bound in the lignin matrix such that in order to stimulate further conversion or reaction of lignin with NaOH, the concentration of NaOH would have to be increased. Considering the total amount of lignin in 1 g of the grass, the delignification process is 73% efficient owing to the ASL in the sample in addition to the unreacted lignin or residual AIL in the Napier grass. The estimated order of the reaction is -0.08 (Fig. 16) and since the order of a reaction cannot be negative, it then implies that the reaction order is approximately zero which is also valid as confirmed by the downward slope of the graph or negative value of k since for a zero order reaction, -rA = k which gives, CA= CAO –kt; where –rA= reaction rate, CA = concentration of lignin in NaOH at time t, CAo = initial concentration of lignin at time t = 0 and k = the velocity constant for the reaction; meaning that at longer times, the reaction is not affected by concentration of NaOH which also confirms that the reaction of lignin and NaOH is pseudo zero order or pseudo fractional order with respect to NaOH as seen for conditions prior steady state condition..
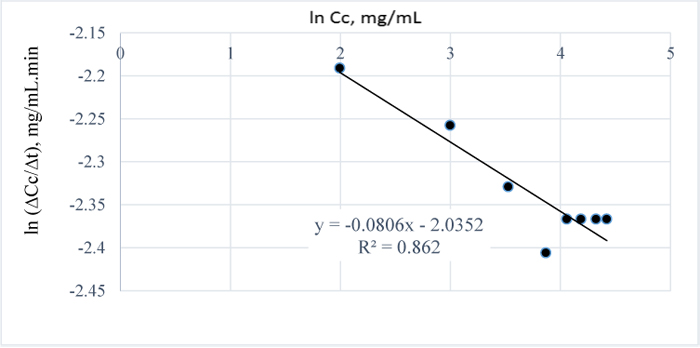 |
Fig. (16). ln (∆Cc/∆t) vs. ln Cc for 3 w/w% NaOH pretreatment at 120°C and optimum time of 17.5 h. |
CONCLUSION
The rate of lignin extraction by NaOH in Napier grass can be accurately described using the Differential Technique combined with the Ostwald Method of Isolation based on the limits of accuracies of the regression lines obtained during the analysis hence, the rate law for the process at the best pretreatment conditions i.e. 3 w/w% NaOH pretreatment of the Napier grass at 120°C is given as –rL = k*CLα where α lies between 0 and 0.2 hence, the reaction of lignin in Napier grass and NaOH obeys a pseudo-fractional order kinetics which may no longer be influenced by NaOH concentration but is only affected by temperature at longer reaction time. The optimum pretreatment time (steady state condition) required for complete removal of AIL in 1 g of the Napier grass sample was found to be 17.5 h. The results show that the delignification process is more energy intensive (i.e. endothermic) with respect to 1 w/w % NaOH pretreatment of Napier grass but less energy intensive (i.e. exothermic) for pretreatment conditions of 3 w/w% NaOH pretreatment of Napier grass. The rate of lignin removal was higher for 3 w/w % than for 1 w/w% NaOH pretreatment of the Napier grass. Also, lignin extraction at 80°C seems to be most influenced by change in NaOH concentration from 1 to 3 w/w% since the highest %difference in lignin concentration in the extract was obtained at 80°C considering reactions between 80 and 120°C, while the %difference was lowest for lignin extraction at 100°C (Table 6); this gives a clue of the weaker influence the change in NaOH concentration has on the lignin in the extraction process hence, the reaction at 100°C seems to be the least influenced by change in concentration since it gives the least %difference or change in lignin concentration for all pretreatment conditions.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors wish to appreciate Covenant University for her sponsorship as well as granting access to facilities when needed. The authors appreciate Sanni Samuel for planning the project, executing it, preparing the manuscript and detailing on the reaction kinetics. Olasubomi Akinrinola for carrying out the experimental works, Esther Yusuf and Omololu Fabgiele for assisting with the analyses and Oluranti Agboola for contributing to the kinetic studies and editing of the manuscript.