RESEARCH ARTICLE
Electrochemical Polymerised Graphene Paste Electrode and Application to Catechol Sensing
Jamballi G. Manjunatha*
Article Information
Identifiers and Pagination:
Year: 2019Volume: 13
First Page: 81
Last Page: 87
Publisher ID: TOCENGJ-13-81
DOI: 10.2174/1874123101913010081
Article History:
Received Date: 12/04/2019Revision Received Date: 28/05/2019
Acceptance Date: 12/06/2019
Electronic publication date: 31/07/2019
Collection year: 2019

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective:
To build up an advantageous strategy for sensitive determination of catechol (CC), a poly (proline) modified graphene paste electrode (PPMGPE) was fabricated and used as a voltammetric sensor for the determination of CC.
Methods:
The performance of the modified electrode was studied using cyclic voltammetric (CV) and differential pulse voltammetric method (DPV). The modified electrode was characterized by CV and DPV. The surface of the modified electrode was examined by FESEM. The electrochemical behavior of CC in phosphate buffer solution (pH 7.5) was inspected using bare graphene paste electrode (BGPE) and PPMGPE.
Results & Conclusion:
The PPMGPE shows a lower limit of detection, calculated to be 8.7×10–7mol L−1 (S/N=3). This modified electrode was applied successfully for the determination of CC in water samples without applying any sample pretreatment.
1. INTRODUCTION
Graphene paste electrode was particularly used for the determination of bioactive molecules because of the easy fabrication of polymer modified electrode, reclaimable and good response and less background current [1-8]. Very few modified electrodes were fabricated by using graphene paste, polymer and surfactants for the analysis of CC by using voltammetric techniques. Catechol is an environmental pollutant which affects the human nervous system and difficult to be degraded in the ecological environment [9-13]. Henceforth, determination of CC from compost bioremediation of municipal solid waste is a basic issue. Moreover, with the enhancement of the requirement for environmental quality, there should be a set of fast and adequate detection technique. In this way, it is important to build up a fast, simple and sensitive way to detect CC. These days, there are some unique approaches to identify CC, including chromatography [14, 15], fluorescence [16], spectrophotometry [17, 21], chemiluminescence [18] and electrochemical method [19, 20]. Tragically, most of them hold the requirements of the expensive instruments and the sample pretreatment, which have the imperfections of the large expense and long time. The technique to polymerization is one of the key issues in developing a reliable biosensor. Polymer modified electrodes have received a great deal of attention as an electrode material because it shows some fascinating properties with regards to the electrochemical investigation [22]. Polymer modified electrodes have been widely applied in the field of electrochemistry attributable to their good stability, permselectivity, novel physical and chemical properties [23]. Studies have demonstrated that polymer modified electrodes show an improved response for the determination of different biological and clinical molecules [24]. Proline can be easily electro-polymerized on the electrode surface to form poly (proline) film. Polymerization film has been utilized in bunches of oxidation and reduction catalytic processes [25-27]. The electrochemical polymerization in different ratios on glassy carbon electrode by cyclic potential sweep has been reported by Yongjun et al. [28]. Polymer modification for enhancing the analytical sensitivity owing to high electron density, dielectric property, catalytic function and incredible biocompatibility [26-29].
Our research team has made progress in researching polymer modified graphene paste electrodes. In this paper, the fabrication of a graphene-based polymer modified biosensor for the determination of CC was portrayed. The polymer modification was performed using cyclic voltammetry from an acidic solution containing appropriate concentrations of monomer and buffer. The polymer modified electrode could remarkably enhance the electrochemical responses of CC and improve the sensitivity of CC detection and its oxidation mechanism (Fig. 1) [35]. Moreover, the electrochemical sensor was effectively utilized to determine the CC concentration in the real sample.
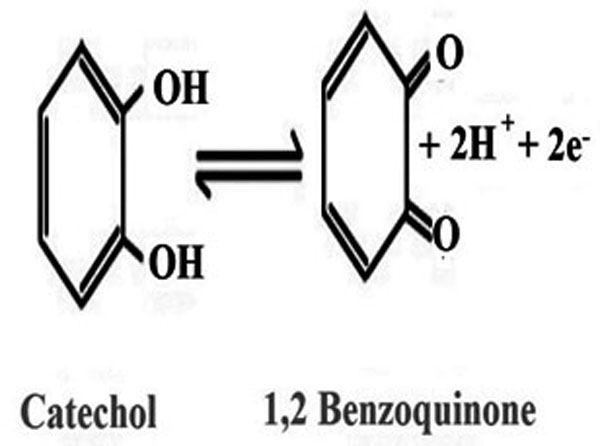 |
Fig. (1). The scheme of oxidation of CC. |
2. MATERIALS AND METHODS
2.1. Reagents and Apparatus
Chemicals and reagents utilized in this work were of analytical grade and were used as received. These were Catechol (SRL, Maharashtra), silicone oil, disodium hydrogen phosphate, monosodium di hydrogen phosphate and SDS obtained from Himedia chemicals, India and were used as received. Graphene (6-8 nm thickness, five μM wide) was obtained from TCI Co. restricted (Japan). Phosphate buffer solution (PBS) was prepared by a combination of an appropriate quantity of NaH2PO4/ Na2HPO4. The stock solution of CC (25×10-4 M), proline (25×10-3 M) were prepared in the double distilled water. All the solutions were prepared by double distilled water.
Voltammetric investigations were carried out on a CHI 6038E electrochemical workstation (USA). All measurements were conducted in a three-electrode cell with a platinum wire as an auxiliary electrode and a saturated calomel electrode (SCE) as a reference electrode. The working electrode was the homemade graphene paste electrode with a diameter of 3 mm modified with poly (proline) film.
2.2. Preparation of the Working Electrodes
The Graphene paste electrode (GPE) was obtained by homogeneous mixing of graphene with silicone oil in a mortar and pestle at a ratio of 60/40 (w/w). The paste was then inserted into the electrode cavity (3mm) and electrical contact was made by means of copper wire. BGPE surface was smoothed on a tissue paper. Tests were performed in 0.1 M PBS of pH 7.5 with various concentrations of CC.
Electrochemical polymerization of Proline is carried out potentiodynamically in the potential window from −0.6 V to +1.2 V for 10 cycles (Fig. 2). While the polymerization process is well-known, the number of cycles and the concentration of proline have been optimized. After polymerization, the electrodes were washed repeatedly with distilled water. Polymerised electrode works as the working electrode for the electrochemical detection of CC.
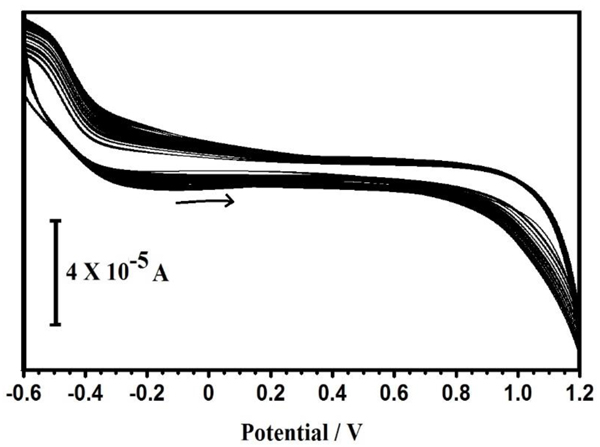 |
Fig. (2). Successive continuous ten cyclic voltammograms for the electrochemical polymerization of 1×10-3 M proline on a BGPE in 0.1 M M PBS (7.5pH) at the scan rate 100 mV/s. |
2.3. Electrochemical Measurements
CC analysis was achieved in a glass cell with a three-electrode configuration. The experiments involving cyclic voltammetry and differential pulse voltammetry (DPV) were performed in the potential range from -0.2 V to 0.6 V and -0.2 V to 0.40 V, respectively. All the experiments were carried out at the room temperature.
3. RESULTS AND DISCUSSION
3.1. Morphological Characterization of BGPE and PGMCPE
The surface morphology of BGPE and PPMGPE was determined by FESEM. Fig. (3) indicates that there are obvious differences between the two electrodes. Morphology of the (a) BGPE and (b) PPMGPE is shown in Figs. (3a) and (b), respectively. Generation of a thin layer on the GPE surface can be proved by the deposited proline clusters on the surface.
3.2. Cyclic Voltammetric Detection of CC at PPMGPE
Fig. (4) shows the cyclic voltammetric responses at BGPE and PPMGPE in 0.1 M PBS solution (pH 7.5) containing 0.1 mM CC. At the BGPE, CC (Fig. 4) 0.1 mM reversible electrochemical behaviors with relatively weak redox currents. The CV of PPMGPE shows well-defined anodic and cathodic peaks at 0.112 V and 0.036 V, respectively, with an enhanced current response due to the good catalysis of PPMGPE for CC oxidation. The total peak current for the oxidation of CC at the PPMGPE is about 178% higher. In comparison to the BGPE, the electrochemical reaction of CC at the PPMGPE shows an outstanding enhancement. In the oxidation peak, reduction peak potential separations of the CC significantly narrowed.
Investigation of CC was done by applying the DPV technique at BGPE and PPMGPE in 0.1 M PBS (pH 7.5) solution containing 0.1 mM CC. As shown in Fig. (5), at the BGPE, the electrooxidation of CC molecules presented weak peaks as the determination of the CC molecules is very difficult. In contrast, at the PPMGPE, well-defined oxidation peaks were observed at 0.06 V and higher (approximately 7.2 times) oxidation peak currents appeared.
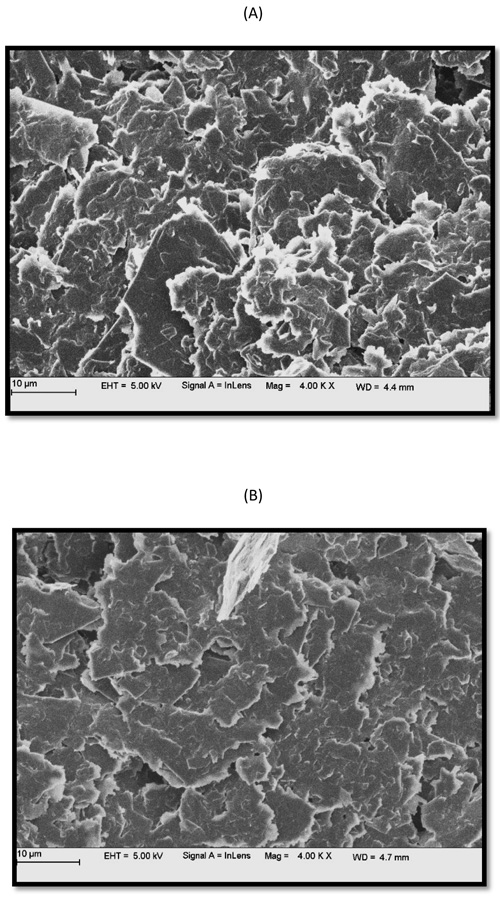 |
Fig. (3). FESEM image of (a) BGPE (b) PPMGPE. |
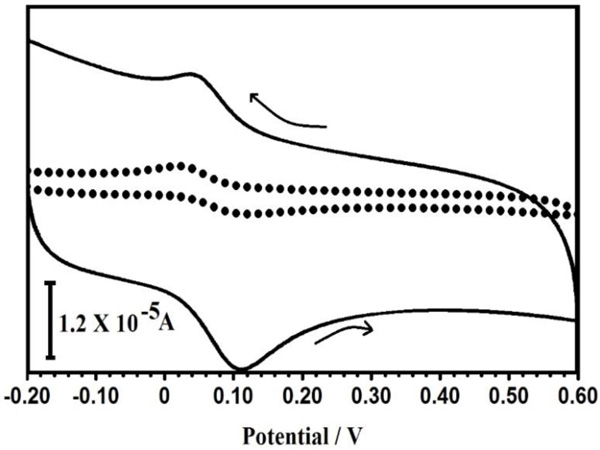 |
Fig. (4). (a) cyclic voltammograms of CC (1×10-4 M) in 0.1 M phosphate buffer solution of pH 7.5 at BGPE (dotted line) and PPMGPE (solid line). |
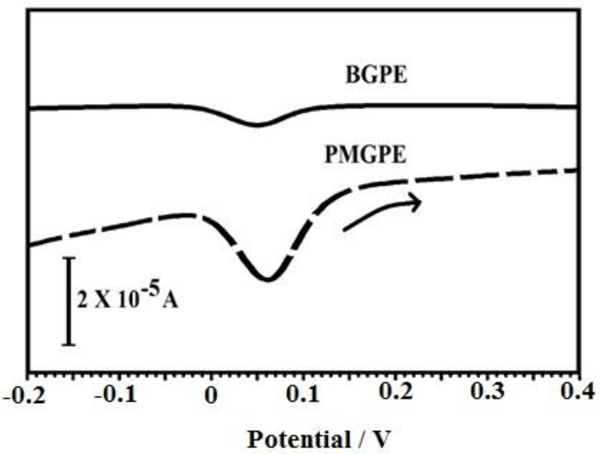 |
Fig. (5). DPVs of a solution containing CC (1×10-4 M) in 0.1 M PBS (pH 7.5) at the BGPE (solid line), the PPMGPE (dashed line). |
Comparing the voltammetric peaks of PPMGPE in the presence and absence of 0.1 mM CC, in 0.1 M PBS solution, at a scan rate 100 mVs−1(Fig. 6), it is revealed that the observed peak is exclusively due to the oxidation of CC and there was no redox peak of PPMGPE in the blank solution. From these measurements, it can be concluded that the background current of the buffer (supporting electrolyte) has no interrupting signal at the time of the electrochemical determinations on the PPMGPE in the potential window between -0.2 V and 0.6 V.
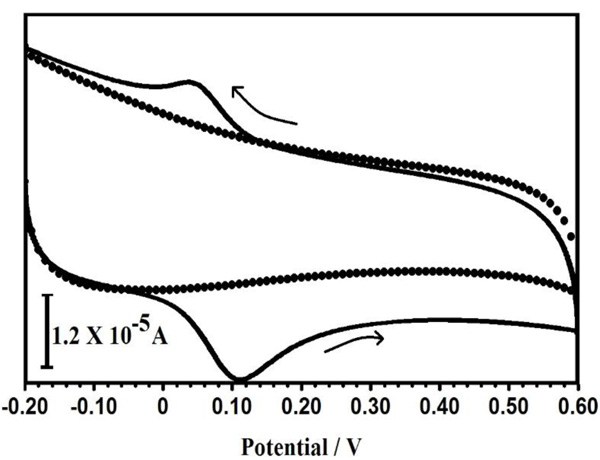 |
Fig. (6). A typical cyclic voltammograms of PPMGPE with CC (1×10-4 M) (solid line) in pH 7.5 PBS without 1×10-4 M CC (dotted line) at pH 7.5 PBS. |
3.3. Measurement of Repeatability, Reproducibility and Stability
The repeatability and reproducibility of the PPMGPE sensor for the determination of CC have been analysed by the CV studies. CVs were recorded in pH 7. 5 PBS at the scan rate of 100 mV/s in the presence of CC at 0.1 mM. The modified sensor indicates excellent repeatability with a relative standard deviation (R.S.D.) of 3.8% for (n = 4) continuous measurements. Moreover, it shows an agreeable reproducibility with an R.S.D. of 2.3% for 3 distinctive determinations.
Stability of the PPMGPE has been evaluated by storing it at the closed vessel. It has been stable for seven days and after that, a gradual decrease (6%) was observed from the current initial value. These values indicate that the modified film was electrochemically active and retain good stability.
3.4. The Effect of Solution pH
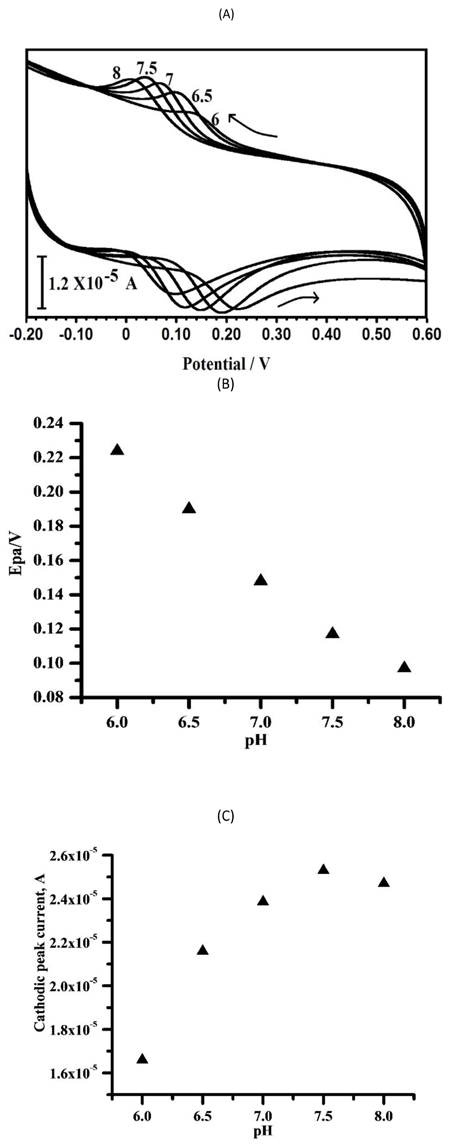
The effect of solution pH on the CV responses of CC using the PPMGPE was measured. As the pH was raised from 6 to 8.0, the oxidation peak potentials of the CC deviate to more negative values as shown in Fig. (7a) with linear relationship equation Epc of CC with pH and were described as Epa (V) = 0.511–0.0631 pH (R = 0.9977) (Fig. 7b). The slope (63.1 mVpH-1) of CC adjacent to the theoretical value of 59 mV pH-1, expresses that their oxidation reactions are followed by the transfer of an equal number of protons and electrons [5-11]. We chose 7.5 as the best pH for the analysis of CC, because of the higher sensitivity at this pH (Fig. 7c).
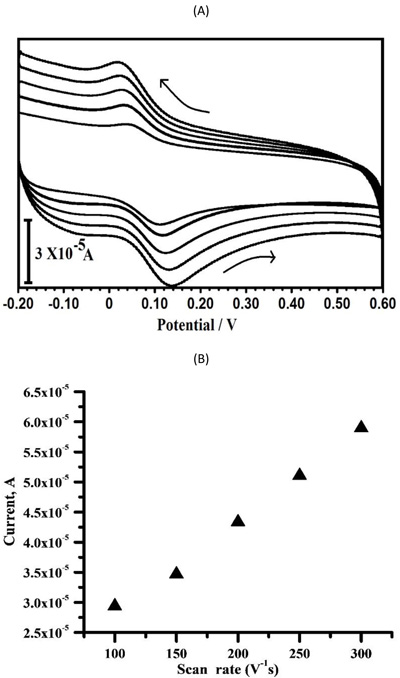
3.5. The Effect of Potential Sweep Rate
The effect of sweep rate or scan rate on the oxidation peak current and peak potential can examine the electrochemical kinetics of electrode reaction [2-4]. Fig. (8a) shows that the CV reaction at the PPMGPE with various scan rates in 0.1 M PBS solution (pH 7.5) consists of 0.1 mM CC. When the scan rate varies from 100 mV/s to 300 mV/s, the oxidation peak currents of CC (Fig. 8b) increase with the changes of scan rate, while their oxidation peak potentials gradually deviate to positive values. Moreover, the relationship between oxidation peak currents and the scan rates is linear. The linear equations are expressed as follows: Ipa (μA) = 13.32+0.150 ν (mVs-1) with correlation coefficient of r2 = 0.9976. The linear relationships show that the CC molecule was influenced by the surface adsorption-controlled process [22-24].
3.6. PPMGPE Electrochemical Sensors for CC Detection
The CV was used to prepare the calibration curve. Under the optimized conditions, the electrode response to CC concentrations was checked at the PPMGPE (Fig. 9). The results showed two calibration curves corresponding to two concentration range of 2×10-6- 4.5×10-5 M and 5 ×10-5-1.5×10-4 M CC. The regression equations were Ipa (A) = 1.34×10-5+0.089 C, (r2=0.999) and Ipa (A) = 1.58×10-5+0.039 C, (r2=0.997), respectively. The limit of detection was obtained (according to DL = 3 sb/m, where sb is the standard deviation of the blank response and m is the slope of the calibration plot) [25-27] as 8.7×10-7 M and limit of quantification obtained as 4.3×10-6 M. Detection limit for CC at PPMGPE is in comparison with other research publication Refs [30-36] while the proposed electrochemical sensor was very easier than other electrochemical sensors (Table 1).
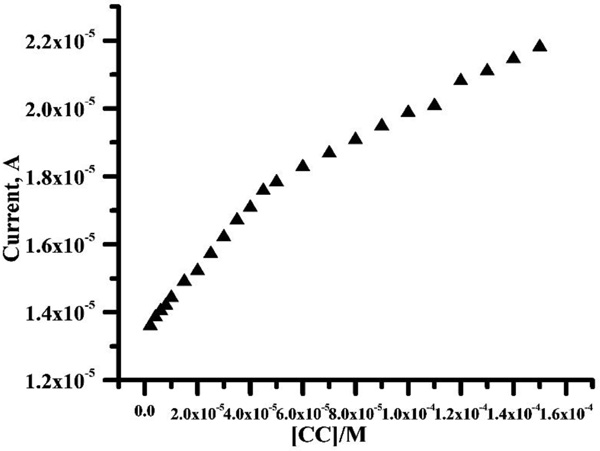 |
Fig. (9). Calibration plot for the determination of CC at the PPMGPE in pH 7.5 PBS with the scan rate 100 mV/s. |
3.6. Real Sample Analysis
To measure the applicability of the PPMGPE in the real sample analysis, CC was used to determine it in water. The standard addition technique was applied for the determination of recoveries. Recoveries have been found to be in between 94.1-97.4%.
|
Working Electrode |
Limit of Dtection in μM |
Method | Reference |
|---|---|---|---|
| Zn/Al Layered Double Hydroxide Film MGCE |
1.2 | DPV | 30 |
| Glassy carbon electrode in CPB and SDBS |
3 | DPV | 31 |
| Silsesquioxane-MCPE | 10 | DPV | 32 |
| Graphene oxide and multiwall carbon nanotubes |
1.8 | DPV | 33 |
| [Cu(Sal-β-Ala)(3,5-DMPz)2] /SWCNTs/GCE |
3.5 | DPV | 34 |
| Poly(calmagite) MCPE | 2.55 | DPV | 35 |
| PEDOT/GO modified Electrode |
1.66 | DPV | 36 |
| PPMGPE | 8.7× 10-7 | CV | This work |
CONCLUSION
In this present work, we have favorably employed an easy methodology for the electrochemical fabrication of PPMGPE. The modified electrode was analyzed by FESEM. This study shows that the prepared proline film was decorated on the electrode. The electrochemical behavior of the PPMGPE has been tested using CV and DPV methods. The PPMGPE possesses the high electroactive surface area which is well appropriate for the determination of CC. Lastly, the PPMGPE is also employed for the detection of CC in real samples. Thus, the PPMGPE exhibits higher stability, reproducibility and promising electrocatalytic activity and quick response towards the determination of CC in both lab and real sample analysis.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The study financially supported by We gratefully acknowledge the financial support from the VGST, Bangalore under Research Project. No. KSTePS/VGST-KFIST (L1)2016-2017/GRD-559/2017-18/126 /333, 21/11/2017.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support from the VGST, Bangalore