All published articles of this journal are available on ScienceDirect.
The Effect of Various Reducing Agents on the Process of Solid-phase Reduction of Highly Phosphorus Ferromanganese Ore
Abstract
Introduction
This paper examines the effect of various reducing agents on the combined reduction of iron and phosphorus from high-phosphorus ferromanganese ores. These ores have a complex mineral composition, making their processing more challenging. Solid-phase reduction is a promising method for converting iron and phosphorus into metallic phases while preserving manganese oxides.
Methods
Experiments were conducted using ferromanganese ore samples at 900 °C in a laboratory furnace. Solid carbon, carbon monoxide (CO), and hydrogen gas served as reducing agents. The phase and chemical composition of the products were analyzed using X-ray phase analysis and electron microscopy.
Results
Solid carbon and CO reduced Fe, P, and Mn to metallic phases. Hydrogen facilitated the reduction of Fe and P while maintaining manganese oxide (MnO) in the oxide form. The hydrogen reduction yielded a higher phosphorus content in the metallic phase compared to carbon or CO. X-ray phase analysis identified α-Fe, MnO, SiO2 and Mn2SiO4 phases.
Discussion
Hydrogen demonstrates high selectivity in reducing Fe and P over Mn. The phosphorus content in the metallic phase was higher with hydrogen compared to carbon or CO. X-ray analysis confirmed the presence of reduced iron and stable oxide phases at 900 °C.
Conclusion
The choice of reducing agent has a significant influence on the phase composition and extraction of target components. Hydrogen gas shows the best results in combined iron and phosphorus reduction, making it a promising candidate for further research and industrial applications.
1. INTRODUCTION
In the context of the growing production of manganese ferroalloys and the depletion of reserves of first-class manganese ores, the search for alternative sources of raw materials is becoming an urgent task [1-3]. One possible solution is the processing of substandard manganese ores, which are currently not utilized in industry due to their low quality and high content of harmful impurities [4-6]. Substandard manganese ores are characterized by high phosphorus and iron content, which makes them unsuitable for direct use in production. However, with comprehensive processing and enrichment, they can serve as a valuable source of manganese [7, 8]. Globally, there is experience in the pyrometallurgical treatment of ferromanganese ores through electric melting to produce low-phosphorus slag. The smelting of low-grade ores into high-quality manganese slag by removing iron is widely practiced in the production of manganese and its alloys [5, 9, 10].
During the metallurgical processing of manganese ores and concentrates, iron can be completely separated by selective reduction, allowing the manganese content in the resulting slag to be increased and the phosphorus content to be reduced. At the same time, up to 15-20% of the manganese contained in the charge passes into the metal. Oxides of CaO, MgO, Al2O3, and SiO2 are almost entirely converted into slag. At the same time, the slag contains about 38-40% Mn and no more than 0.012-0.017% Pp, and the associated phosphorous alloy contains %: 50-55 Mn; 40-45 Fe; 2.5-4.5 Pp; 1.5-2.5 Si; 2.5-3% C [11]. Such a technological scheme for the production of phosphorus-standard manganese ferroalloys remains an indispensable, yet very inefficient, metallurgical cycle. Its main disadvantage is that the process is energy-intensive, very costly, and results in high manganese losses.
A pyrometallurgical method for dephosphorization of manganese-containing products was proposed at the A.A. Baykov Institute of Metallurgy and Materials Science [12-14]. The authors of the proposed method claim that it allows phosphorus to be removed without loss of manganese by using gaseous carbon monoxide CO as a reducing agent. To achieve this, the manganese ore or concentrate is melted in an electric arc furnace, raising the melt temperature to 1000-1800 °C to dephosphorate the melt, and carbon monoxide is purged through the manganese-containing oxide melt. In this case, phosphorus from the oxides is reduced by gaseous carbon monoxide, and the reduced gaseous phosphorus is removed with waste gases. The degree of dephosphoration is more than 80%. However, this method is still not used in the production of manganese alloys.
It is also known that the accompanying elements of the raw material affect the temperature of manganese reduction from oxides [15]. In particular, the reducing agent and the presence of iron oxides are of great importance. Similar to the rate at which carbon monoxide is removed from the system, variations in vacuum conditions, atmospheric composition, and other experimental parameters contribute to discrepancies in the reported temperatures at which manganese reduction begins, according to different authors. It should be noted that in the process of processing iron-containing manganese ores using pyrometallurgical methods, carbon acts as an iron reducing agent. However, the use of carbon in this process complicates the technological process due to the reduction of active metals and the formation of their carbides [16-18]. Hydrogen can serve as an alternative reducing agent for iron in the processing of iron-bearing manganese ores. Studies have examined the reduction of iron using hydrogen-containing gases from rich hematite and magnetite ores [19-24]. It has been found that hydrogen has several advantages over carbon-containing reducing agents, which are particularly important in the selective extraction of metals from complex ores [25]. Nevertheless, the processes occurring during the reduction of iron with hydrogen from complex iron-containing manganese ores have not been sufficiently studied. Additional research is needed to develop and master new recycling technologies. First and foremost, hydrogen enables a cleaner reduction process, as the by-product of the reaction is water vapor rather than carbon dioxide, making the process more environmentally friendly and aligned with the goals of decarbonizing the metallurgical industry. Additionally, hydrogen exhibits a high reducing potential and can selectively reduce iron without affecting manganese, a property that is particularly important when processing ferromanganese ores. This selectivity allows for better control over the phase composition of the final products and reduces the energy required for subsequent separation of components. Moreover, hydrogen facilitates a decrease in the reduction temperature, which has a positive impact on both the economic and energy efficiency of the production process.
The aim of this study is to conduct a comparative investigation of the combined reduction of iron and phosphorus from ferromanganese ores using solid carbon, carbon monoxide, and hydrogen, with the goal of producing a manganese oxide concentrate.
2. MATERIALS AND METHODS
The object of the study is the ferromanganese ore of the Selezenskoye deposit (Russia), characterized by relatively high content of iron and phosphorus. Fig. (1) shows a general view of the ferromanganese ore of the Selezenskoye deposit.

General view of ferromanganese ore.
A low manganese content and a significant amount of harmful impurities, including phosphorus and iron, characterize the ferromanganese ore of the Selezenskoye deposit. The ore contains a variety of minerals typical of manganese and ferromanganese deposits, with quartz being the predominant phase. Prior to the reduction experiments, the raw ore was crushed to a particle size of less than 1 mm to ensure a more homogeneous chemical composition.
Experiments on the reduction of iron and phosphorus from ferromanganese ore with solid carbon or carbon monoxide were conducted in a sealed furnace equipped with a graphite heater [26, 27]. For this, two corundum crucibles were simultaneously placed in the furnace's working space. The first crucible was filled with a mixture in the form of powder with grains of 1 mm in size of ferromanganese ore and a mixture of graphitized electrodes. Ore powder was placed in the second crucible, but without a solid reducing agent. The furnace was covered with a lid to create a reducing atmosphere, heated to 900°C, and maintained for 90 minutes. After the experiments were completed, the furnace was cooled to room temperature. The recovered sample, to which solid carbon was added, was sieved to remove the reducing agent. Experiments on the combined selective solid-phase reduction of iron and phosphorus using hydrogen were conducted in a vertical furnace reactor manufactured by RB Automazione, model MM6000. The technique and scheme of the furnace working areas are described in detail in the work previously published by the authors [28]. During the recovery process, the furnace was purged with hydrogen at a temperature of 900 °C for 30 minutes, with a hydrogen consumption rate of 5 liters per minute.
The recovered samples and the initial sample of ferromanganese ore were filled with epoxy resin. They were then sanded in a Struers Tegrapol-15 installation and examined using an optical microscope in reflected light. Microrentgenospectral analysis of the samples was performed using a JSM-6460LV electron microscope equipped with wave and energy-dispersive analyzers. X-ray diffraction phase analysis of the samples was conducted on a Rigaku Ultima IV X-ray diffractometer. The results were processed using the “Match” software.
3. EXPERIMENTAL RESULTS AND THEIR DISCUSSION
The appearance of ferromanganese ore, as studied and used in our experiments, is shown in Fig. (2). The ore has a characteristic color and texture that makes it easy to distinguish from other types of rocks.
According to micro-X-ray spectral analysis, the color of the grains of ferromanganese ore is heterogeneous, varying from gray to brownish with inclusions of oxides Mg, Al, Si, K, Ca, Ba (Fig. 2). According to the results of microrentgenospectral analysis, ferromanganese ore has a rather complex textural and structural pattern. In the spectra of particles 1 and 2, iron oxides (the atomic fraction of iron is 53.8 – 55.9%) with a small amount of manganese and silicon are detected. The presence of barium oxide along with manganese oxides is also observed in the ore (Table 1).
In the course of previous experiments, it was revealed that the initial ore contains elements such as iron, silicon, magnesium, aluminum, phosphorus, oxygen, and calcium. However, only oxygen, iron, manganese, and silicon are present in significant amounts [26, 27]. Studies of ferromanganese ore samples reduced simultaneously and under identical conditions using solid carbon or gaseous carbon monoxide show that, at the same temperature, the reducing agent influences which elements enter the metallic phase: Fe and P in a CO atmosphere, or Fe, P, and Mn when in contact with solid carbon. According to the results of expe>riments using hydrogen as a reducing agent at a tempe>rature of 900 ° C and exposure for 30 minutes, only Fe and P pass into the metal part; the higher manganese oxides are reduced to monoxide and are completely pre>served in the oxide phase. The content of phosphorus reduced by hyd>rogen in the metal is significantly higher than in samples reduced in an atmosphere with CO or solid carbon (Fig. 3, Table 2). This indicates a relatively higher rate of hydrogen reduction of Fe and P.
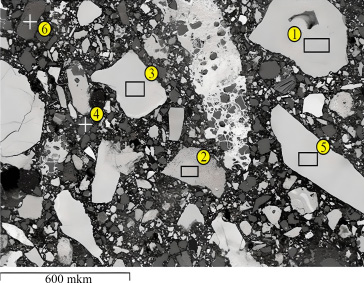
Ore grains and nonmetallic phase of ferromanganese ore of the selezen deposit.
| Place of Analysis |
O | Mg | Al | Si | P | K | Ca | Mn | Fe | Ba | Phases |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Square 1 | 44 | 0.0 | 0.2 | 1.7 | 0.2 | 0.0 | 0.0 | 0.3 | 53.8 | 0.0 | Ore grain |
| Square 2 | 42 | 0.0 | 0.6 | 0.8 | 0.0 | 0.0 | 0.0 | 0.5 | 55.9 | 0.0 | Ore grain |
| Square 3 | 42 | 0.0 | 0.8 | 0.0 | 0.1 | 2.2 | 0.0 | 53.4 | 0.0 | 1.9 | Ore grain |
| Point 4 | 54 | 0.2 | 17.7 | 26.5 | 0.0 | 0.0 | 0.0 | 0.5 | 1.5 | 0.0 | Nonmetallic phase |
| Square 5 | 42 | 0.0 | 0.8 | 0.0 | 0.1 | 2.2 | 0.0 | 53.4 | 0.0 | 1.9 | Ore grain |
| Point 6 | 61 | 14.8 | 0.0 | 0.0 | 0.0 | 0.0 | 23.2 | 0.5 | 0.5 | 0.0 | Nonmetallic phase |
| Reducing Agent | Point of Analysis |
The Content of the Elements, in terms of Mass.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Si | Са | Р | Mn | Fe | Cu | Ba | ||
| Hydrogen | 1 а | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 2.4 | 97.3 | 0.0 | 0.0 |
| 2 а | 55.5 | 0.0 | 5.0 | 0.7 | 0.0 | 36.5 | 1.0 | 0.0 | 1.3 | |
| 3 а | 56.3 | 0.0 | 0.0 | 0.2 | 0.0 | 42.9 | 0.0 | 0.6 | ||
| 4 а | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 | 0.0 | 98.9 | 0.0 | 0.0 | |
| 5 а | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 1.2 | 98.6 | 0.0 | 0.0 | |
| Solid carbon | 1 б | 57.2 | 2.8 | 0.0 | 0.0 | 0.0 | 39 | 0.0 | 0.0 | 1.0 |
| 2 б | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 1.1 | 97.8 | 1.0 | 0.0 | |
| 3 б | 57.6 | 0.0 | 0.0 | 0.0 | 0.0 | 31.6 | 10.5 | 0.0 | 0.4 | |
| Carbon monoxide | 1 в | 51.4 | 0.0 | 0.0 | 0.0 | 0.2 | 48.4 | 0.0 | 0.0 | 0.0 |
| 2 в | 53.9 | 0.0 | 0.0 | 0.0 | 0.1 | 46 | 0.0 | 0.0 | 0.0 | |
| 3 в | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 99 | 0.9 | 0.0 | |
| 4 в | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 98.8 | 0.6 | 0.0 | |

Type and composition of phases in ferromanganese ore after reduction firing: a – at 900 ° C and exposure for 30 minutes in an atmosphere of hydrogen; b – at 900 ° C and exposure for 90 minutes in contact with solid carbon; c – at 900 ° C and exposure for 90 minutes in an atmosphere of CO.
The results of the study of the distribution of iron and manganese between phases during solid-phase reduction are confirmed by the data presented on the concentration maps of these elements in the reaction products (Fig. 4). Analysis of these maps allows us to conclude that during the reduction of hydrogen in the atmosphere, almost all iron passes into the metallic phase, while manganese remains outside the metal particles. The distribution of phosphorus on the maps appears to be uniform and does not show any obvious patterns, which is probably due to its low concentration.
Fig. (5) shows diffractograms of samples after reduction firing in an atmosphere of hydrogen and carbon dioxide at a temperature of 900 °C. The results of X-ray phase analysis show that the following phases are present in the samples: α—Fe — iron; MnO — manganese monoxide; SiO2 — quartzite; Mn2SiO4—tephroite in a sample reduced in a carbon dioxide atmosphere. No phosphorus compounds were detected on the diffractograms, which suggests that they are in solution with iron. According to X-ray phase analysis, the main phases of the initial ore are MnO2, SiO2, Fe2O3, CaCO3, FeCO3, and phosphorus is concentrated in minerals such as Ca(PO3)2 and Fe4H3P3O15.
3.1. Phases
1–MnO2, 2–SiO2, 3– MnO, 4–Fe2O3, 5 – CaCO3, 6 – FeCO3, 7 – Са(РО3)2, 8–Fe4H3P3O15, 9- α-Fe, 10- Mn2SiO4.
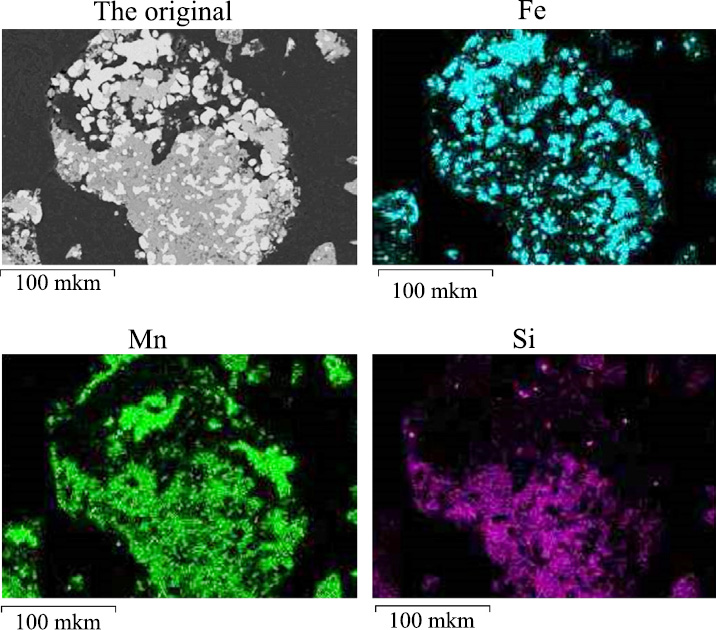
Map of the distribution of elements after reduction in the atmosphere of hydrogen at 900°C for 30 minutes.
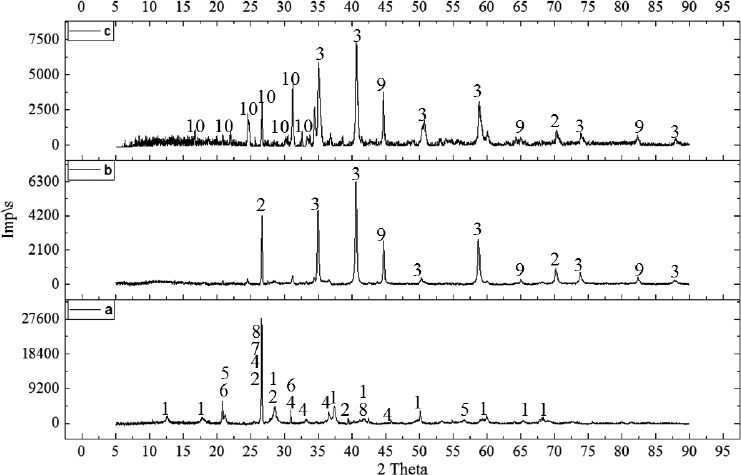
X-ray phase analysis of the initial sample (a), after reduction by hydrogen (b) and in an atmosphere of CO (c).
CONCLUSION
- As a result of reduction roasting in a hydrogen atmosphere at 900 °C for 30 minutes with a hydrogen flow rate of 5 L/min, it was demonstrated that iron with a high phosphorus content can be obtained while largely preserving manganese oxides in the slag phase.
- When using hydrogen as a reducing agent, the content of reduced phosphorus in the metal is significantly higher than in the samples recovered in a CO atmosphere or in contact with solid carbon, which indicates a higher rate of reduction of these elements by hydrogen.
- Experimental results have shown that hydrogen can be an effective reducing agent for processing complex, highly phosphorus iron-containing manganese ores in order to obtain iron and a concentrate of oxides of the second metal.
AUTHORS’ CONTRIBUTIONS
The authors confirm contribution to the paper as follows: N.K.: Data Analysis or Interpretation; B.S: Data Collection; G.A.: Visualization; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| Iron | = α-Fe |
| Manganese monoxide | = MnO |
| Quartzite | = SiO2 |
| Tephroite | = Mn2SiO4 |
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
ACKNOWLEDGEMENTS
Declared none.


