All published articles of this journal are available on ScienceDirect.
Effects of Conjugated Linoleic Acid on Oleogel Structure and Oxidative Stability
Abstract
Background
Oleogels have attracted attention as healthier alternatives to traditional solid fats. Conjugated linoleic acid (CLA) is valued for its bioactive effects; however, integrating CLA into oleogels and ensuring their stability remains challenging. Beeswax (BW) is a widely used organogelator, yet CLA-enriched oleogels with BW have not been previously explored. This study aimed to prepare and characterize CLA-enriched oleogels using beeswax, focusing on their physical, thermal, rheological, and oxidative stability properties.
Methods
CLA was chemically synthesized from linoleic acid and incorporated into sunflower oil at concentrations of 25% and 50%. BW was used as the oleogelator at 2%, 3%, and 4%. Oleogels were prepared via heating and controlled cooling. Physicochemical analyses included oil binding capacity, gel stability, color, rheology, texture, X-ray diffraction (XRD), and thermo-oxidative stability. Storage stability was monitored over 120 days at 25°C by measuring peroxide value (PV) and thiobarbituric acid reactive substances (TBARS).
Results
Oleogels exhibited strong oil-binding capacity and viscoelastic behavior. Increasing BW concentration enhanced structural firmness, while higher CLA content significantly improved oxidative stability but reduced thermal resistance (Tonset and Tmax). XRD analysis revealed predominantly α- and β′-type crystals originating from BW. CLA-enriched oleogels showed significantly lower PV and TBARS values during storage compared to controls, particularly in formulations with moderate CLA levels and ≥3% BW.
Discussion
CLA’s antioxidant properties contributed positively to oleogel oxidative stability; however, excessive CLA concentrations adversely affected thermal resistance and gel homogeneity. BW effectively structured the gels and facilitated CLA integration. The interaction between CLA and BW influenced crystal formation, firmness, and storage stability. Study limitations include the use of a single oil type and absence of sensory evaluation or thermal cooking simulations.
Conclusion
CLA-enriched BW oleogels represent a promising alternative to traditional solid fats by combining biofunctional benefits with mechanical and oxidative stability at room temperature. Their incorporation into food systems may reduce dependence on unhealthy fats while extending shelf life. Future research should investigate sensory properties, culinary performance, and broader food applications.
1. INTRODUCTION
Edible oils are classified as solid, semi-solid, or liquid based on their structural properties at room temperature. The chemical composition of the oils influences these physical states and determines their specific applications. For instance, oils that remain completely liquid at room temperature are commonly used as salad oils, while semi-solid fats are utilized as spreadable margarine for breakfast. Solid fats, such as margarine and shortenings, are essential components in bakery products. Shortenings play a vital role in bakery applications by delaying staling, retaining gas, supporting dough rise, improving texture, providing lubrication, and enhancing flavor. Today, with the increased consumption of unhealthy and unbalanced diets through ready-made foods, diseases such as obesity, cardiovascular diseases, and digestive system disorders have become more prevalent. Consequently, people resort to lean diets, and due to uncontrolled eating habits, more significant health problems arise. Fats and oils are fundamental components of human nutrition. To ensure the proper utilization of fat-soluble vitamins and meet the functional needs of essential fatty acids, fat intake should not be excessively reduced. On the other hand, excessive consumption of saturated and trans fatty acids has been reported to lead to health problems such as obesity, metabolic syndrome, and cardiovascular diseases. For this reason, oleogel production has been advanced through the organogelation technique, a novel structuring method considered a promising alternative to traditional fat modification processes. Waxes from various sources have been used to obtain oleogels [1, 2].
In the preparation of oleogels, the type of oil used, along with the type and concentration of the oleogelator, directly affect the textural, rheological, and thermal properties of the oleogels. Furthermore, it has been determined that the type of oil influences the rheological, textural, and thermal properties of the final product, and oils with high levels of unsaturated fatty acids exhibit a stronger gel structure [3].
Conjugated linoleic acids (CLAs) isomers of linoleic acid (LA), are predominantly found in the meat and milk of ruminant animals and their products. CLAs are produced by microbial bio-hydrogenation in the rumen or enzymatic desaturation in the mammary glands and adipose tissues. The Food and Drug Administration (FDA) categorizes CLA as GRAS (Generally Recognized as Safe) for certain foods, such as yogurt, milk, soy milk, and fruit juices. CLAs have attracted attention as biologically beneficial functional lipids. Furthermore, CLAs have been found to positively affect the immune system, improve the LDL/HDL ratio in rabbits, and have beneficial effects on obesity [4, 5].
On the other hand, it was predicted that since CLA has a higher melting point than LA [5] and possesses antioxidative properties [6], it could act synergistically with the oleogelator to prevent oxidative deterioration during the storage process. While many studies have focused on the preparation of oleogels and the production of CLA, CLA-enriched oleogels have not been extensively investigated. Therefore, the present study aimed to produce CLA-enriched oleogels using a mixture of sunflower oil, beeswax (BW), and CLA at varying concentrations and to determine their physicochemical properties and stability during storage.
2. MATERIALS AND METHODS
2.1. Materials
Commercial sunflower oil was purchased from a local market. A fatty acid methyl ester mixture (C4–C24) obtained from Supelco (Bellefonte, PA, USA) was used as an external standard. All other chemicals, including BW, were supplied by Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
2.2. Fatty Acid Composition of Sunflower Oil
The fatty acid methyl esters of sunflower oil were prepared following IUPAC Method 2.301 [7] and analyzed using a gas chromatograph (Shimadzu GC-2010, Japan) equipped with a flame ionization detector (FID) and a DB23 column (30 m length, 0.25 mm internal diameter, 0.25 μm film thickness; J&W). The injector, column, and detector temperatures were set to 240°C, 185°C, and 250°C, respectively. Helium was used as the carrier gas at a flow rate of 0.5 mL/min with a split ratio of 1:90. This analysis was repeated three times.
2.3. Production of CLA
CLA was produced following the method described by Erinç and İşler [8]. LA (25 g) was mixed with KOH (13 g in 25 mL ethylene glycol) and isomerized at 180°C for 8 hours using a heater. Subsequently, ethanol (40 mL) was added, and the pH was adjusted to below 2 by adding 25 mL of 6 N HCl. The resulting product was sequentially rinsed with 80 mL of pure water, 80 mL of hexane, and 80 mL of 30% methanol. Finally, residual solvents were removed using a rotary evaporator (Heidolph Hei-Vap, Germany).
2.4. Amount and Isomer Analysis of CLA
The CLA methyl esters were obtained from the CLA mixture according to Erinç and İşler [8]. In summary, fatty acid methyl esters (FAMEs) were prepared by adding 125 μL of sulfuric acid (1% w/v in methanol) to an oil sample (4 mg) and incubating the mixture at 70°C for 2 hours. Subsequently, 300 μL of sodium chloride solution (5% w/v) was added, and the mixture was further incubated at 70°C for 10 minutes. The reaction mixture was then extracted twice with 30 mL of hexane using a separation funnel. Finally, 250 μL of potassium bicarbonate solution (4% w/v) was added to the extract to obtain the FAMEs.
The separation of CLA methyl esters was performed using an Ag+-HPLC system. A high-performance liquid chromatography system (Shimadzu LC-20A/Prominence) equipped with a UV-VIS detector (Shimadzu SPD-20A) set at 233 nm was utilized. Two analytical columns (ChromSpher 5 Lipids, Ag+-impregnated, 4.6 mm i.d. × 250 mm, stainless steel, 5 μm particle size; Chrompack, Bridgewater, NJ) were connected in series. The mobile phase consisted of 0.1% acetonitrile in hexane, operated isocratically at a flow rate of 1.0 mL/min [9]. Standard CLA solutions (9t-11t, 9c-11t, and 10t-12c isomers) were analyzed under the same conditions to identify CLA isomers. This analysis was repeated three times.
2.5. Preparation of Oleogels
Before preparing the oleogel, the minimum amount of BW required for gel formation was determined. The mixture of BW, sunflower oil and/or CLA was placed in a falcon tube at a total weight of 15.0 ± 0.01 g. This mixture was then heated at 85°C and cooled to room temperature (+25°C) at a cooling rate of 3–3.2°C/min. The test began with 0.5% (w/w) BW and the amount was gradually increased up to 4% in 0.5% increments. The concentration at which the samples no longer exhibited gravity flow was defined as the gelling concentration. After determining the minimum BW concentration for gelation as 2%, the method described by Erinç and Okur was modified and used in oleogels preparation. For this purpose, sunflower oil, BW (2, 3 and 4%) and CLA (25-50% of CLA content of sunflower oil) were weighed in a 250 mL beaker [2]. The beaker was heated in a water bath at 90°C and stirred at a constant speed (200 rpm) for 30 min to obtain homogeneous transparent mixtures. Then, the mixtures were cooled at 25±0.5°C for 1 hour for gelation. Each oleogel formulation was produced in two independent batches.
2.6. Physicochemical Characterization of Oleogels
2.6.1. Oil-binding Capacity (OBC)
OBC of the samples was measured according to Blount et al. [10]. For this analysis, 5 mL of each oleogel sample was melted at 90°C and transferred into pre-weighed Falcon tubes (initial weight: a). The samples were then conditioned at +4°C in a refrigerator for 1 hour. Subsequently, the tubes were reweighed (b), centrifuged at 9,000×g for 15 minutes at 25°C, and allowed to drain for 15 minutes to collect the released oil. The remaining sample was weighed (c), and OBC of the oleogels was calculated using Eq. (1). Each analysis was performed in triplicate.
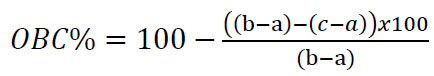 |
(1) |
2.6.2. Gel Stability
Falcon tubes (15 mL) containing 5 g of oleogel were stored in a refrigerator at 4°C for 24 hours, followed by centrifugation at 1,300 × g for 15 minutes at 25°C. Oleogels that exhibited no structural degradation after centrifugation were classified as stable [2]. Gel stability for each sample was tested in triplicate.
2.6.3. Color
The color values of the oleogels were measured using a Minolta Chroma Meter CR-400 (Konica Minolta, Osaka, Japan). Measurements were taken from samples placed in glass beakers, and the L*, a*, and b* values were reported as the average of four readings.
2.6.4. Rheological and Textural Properties
Rheological measurements of the oleogels were performed using a Kinexus Pro rheometer (Malvern Instruments Ltd., Malvern, UK). Oscillation frequency sweep tests were conducted using a parallel plate geometry (20 mm diameter) with a gap of 50 μm at 25°C across a frequency range of 0.01 to 100 s−1 with 10 points per decade. Samples were analyzed immediately after removal from the refrigerator (+4°C), with at least two replicates. The complex shear modulus (G*)/frequency, storage modulus (G′), and loss modulus (G″) were recorded using rSpace for Kinexus Pro version 1.73 (Malvern Instruments Ltd., Malvern, UK).
The firmness of the oleogels was determined using a texture analyzer (TA-XTPlus, Stable Micro Systems Ltd., Godalming, Surrey, UK) equipped with a TTC Spreadability Rig HDP/SR, based on the 'Measure Force in Compression' method. Test conditions included a 5 kg load cell, a test speed of 3.0 mm/s, and a compression distance of 16 mm. Each sample was analyzed in triplicate.
2.6.5. X-Ray Diffraction
X-ray diffraction (XRD) analyses of all samples were performed using a PANalytical Empyrean diffractometer (PANalytical, Malvern, UK) equipped with CuKα radiation and operated at 40 kV and 40 mA. Samples were scanned at a speed of 1°/min. Each sample was measured in triplicate.
2.6.6. Thermo-oxidative Stability
The thermo-oxidative behaviors of the oleogels were determined using Linseis STA PT1600 (Selb, Germany) under constant dry air flow. The samples (10-15 mg) were heated from 25–500 °C with a heating rate of 10 °C/min. For each sample, the analysis was performed three times.
2.7. Storage Stability of Oleogels
The oleogels were stored at 25°C in the dark for 120 days, and thiobarbituric acid (TBA) and peroxide value (PV) measurements were performed every 15 days in accordance with AOCS methods [11]. Sunflower oil (Control-0) and CLA-enriched oils without BW were stored as control samples. All analyses were conducted in triplicate.
3. RESULTS AND DISCUSSION
3.1. Fatty acid Composition
The fatty acid composition of sunflower oil (Control-0), CLA, and CLA mixtures is presented in Table 1. The fatty acid composition of the sunflower oil (%) was as follows: 0.08 ± 0.01% myristic acid, 5.82 ± 0.11% palmitic acid, 0.12 ± 0.01% palmitoleic acid, 3.25 ± 0.06% stearic acid, 35.99 ± 0.72% oleic acid, 54.69 ± 1.04% linoleic acid, and 0.06 ± 0.01% linolenic acid. As shown in the results, the CLA sample, chemically synthesized from pure linoleic acid (LA), contained 87.53 ± 1.10% CLA isomers, consistent with our previous study [8]. By mixing sunflower oil and CLA sample, Control-25 and Control-50 were obtained, containing 25.03±0.52% and 50.07±0.75% CLA, respectively.
| Fatty Acids | CLA Mix | Sunflower Oil | CLA-25 | CLA-50 |
|---|---|---|---|---|
| Miristic acid (C14:0) | nd | 0.08±0.01 | 0.06±0.01 | 0.04±0.01 |
| Palmitic acid (C16:0) | nd | 5.82±0.11 | 4.16±0.08 | 2.89±0.05 |
| Palmitoleic acid (C16:1) | nd | 0.12±0.01 | 0.09±0.01 | 0.05±0.01 |
| Stearic acid (C18:0) | nd | 3.25±0.06 | 2.32±0.05 | 1.39±0.03 |
| Oleic acid (C18:1) | nd | 35.99±0.72 | 25.70±0.56 | 15.40±0.36 |
| Linoleic acid (C18:2) | 12.47±0.01 | 54.69±1.04 | 42.62±0.82 | 30.54±0.55 |
| Linolenic acid (C18:3) | nd | 0.06±0.01 | 0.04±0.01 | 0.03±0.01 |
| 9c-11t CLA | 40.31±0.51 | nd | 11.53±0.24 | 23.05±0.35 |
| 10t-12c CLA | 40.81±0.4 | nd | 11.67±0.26 | 23.34±0.37 |
| Other CLA | 6.41±0.05 | nd | 1.83±0.03 | 3.66±0.04 |
| Total CLA | 87.53±1.10 | nd | 25.03±0.52 | 50.07±0.75 |
3.2. Physicochemical Properties
No gel formation was observed at BW concentrations between 0.5% and 1.5%. Once 2% BW was identified as the minimum concentration required for gelation, oleogels containing 2%, 3%, and 4% BW were formulated and subjected to detailed physicochemical analyses.
3.3. Oil-binding Capacity
OBC is a critical parameter influencing the physical characteristics of oleogels, with higher OBC values indicating improved structural stability [2]. In this study, the OBC of BW oleogels increased significantly (P < 0.05) with rising BW concentration, consistent with the findings of Okur and Erinç [2] and Sarkisyan et al. [12]. The lowest OBC (87%) was observed in oleogels containing 2% BW, whereas the highest OBC (99%) was recorded in those containing 4% BW. However, the CLA concentration did not significantly influence OBC values (P > 0.05) (Table 2).
3.3.1. Gel Stability
Although oleogels were successfully prepared using 2% BW, they did not demonstrate adequate resistance in the centrifugal stability test. Consequently, a minimum BW concentration of 3% is required to achieve a stable gel structure. Furthermore, no significant differences were observed between oleogels containing 25% and 50% CLA (Table 2).
3.3.2. Color
In the color analysis, brightness decreased with increasing BW concentration (2% to 4%), leading to the formation of opaque oleogels. Conversely, the yellowness and greenness of the oleogels increased. However, while the CLA content (25–50%) did not significantly affect the brightness or greenness of the oleogels, yellowness increased (P >0.05) (Table 2).
3.3.3. Thermo-oxidative Stability
Previous studies have demonstrated that oleogelator concentrations significantly influence the thermal behavior of oleogels [3, 13]. Therefore, the thermal properties of oleogels with varying concentrations of BW and CLA were characterized under a dry air atmosphere. The initial decomposition temperature (Tonset) and the temperature at the maximum rate of weight loss (Tmax) for all samples are presented in Table 2.
The Tonset and Tmax values of beeswax were lower than those of the oleogels. Furthermore, as the BW concentration increased, both the Tonset and Tmax of the oleogels decreased. Conversely, the thermo-oxidative stability of the oleogels was negatively affected by increasing CLA content. Specifically, the Tonset and Tmax values decreased as the CLA concentration was increased from 25% to 50%. Among the samples, CLA-25-2 exhibited the highest resistance to thermal treatment, while CLA-50-4 showed the lowest stability.
3.3.4. X-Ray Diffraction
X-ray diffraction (XRD) analysis was conducted to investigate the polymorphism and internal structures of oleogel samples, with the d-spacing of the crystals calculated based on the 2θ values (Fig. 1). The crystal type of the gels is a critical factor influencing physical properties such as plasticity and consistency. As shown in Fig. (1), BW exhibits three diffraction peaks at 19.41°, 21.53°, and 23.89°, corresponding to d spacings of 4.59, 4.14, and 3.74 Å, respectively. These peaks indicate the presence of β, α, and β′ crystals in the structure. In the X-ray diffraction patterns of the oleogel samples, two diffraction peaks at 4.14 and 3.74 Å are clearly visible, confirming that the crystal structure is predominantly attributed to BW. The oleogels are formed through the crystallization of beeswax molecules. These findings are consistent with the results reported by Zhang et al. [14] regarding the crystal types of natural wax-based oleogels.
While the sharp peak at 4.14 Å indicates that all beeswax oleogels, regardless of the CLA and BW proportions, predominantly consist of α crystals, the low-intensity peak at 3.74 Å suggests the presence of β′ crystals. These observations are consistent with the findings reported by Zhang et al. [14]. However, diffraction peaks corresponding to β crystals were not detected, indicating that the oleogel structures do not contain significant amounts of β crystals.
| Samples | CLA-25*-2** | CLA-25-3 | CLA-25-4 | CLA-50-2 | CLA-50-3 | CLA-50-4 | Beeswax |
|---|---|---|---|---|---|---|---|
| OBC (%) | 87.43±0.12a | 98.24±0.13b | 99.64±0.14b | 87.53±0.12a | 98.82±0.12b | 99.73±0.13b | - |
| Stability | - | + | + | - | + | + | - |
| L* | 39.11±0.27c | 34.55±0.28b | 31.26±0.85a | 38.88±0.31c | 33.70±0.36b | 30.31±0.23a | - |
| a* | -1.18±0.02c | -2.50±0.13b | -3.86±0.05a | -1.13±0.05c | -2.48±0.20b | -3.80±0.14a | - |
| b* | 7.00±0.35a | 8.98±0.22b | 10.79±0.10c | 8.45±0.65b | 11.30±0.36d | 14.04±0.06e | - |
| Tonset (°C) | 323.3±0.5f | 319.2±0.3e | 312.4±0.7c | 319.4±0.4e | 314.2±0.5d | 308.6±0.6b | 262.6±0.5a |
| Tmax (°C) | 403.2±0.5g | 395.3±0.7f | 361.5±0.4c | 392.3±0.5e | 384.1±0.5d | 353.2±0.6b | 337.1±0.6a |
Overall, the diffraction patterns of all oleogels were similar, indicating that the crystal structure is not dependent on the ratio of CLA. Furthermore, for all the peaks resolved in the X-ray diffraction patterns, the intensity of each peak in oleogels with 4% BW was higher than that in oleogels with 2-3% BW. The higher intensity of the long-spacing peaks in oleogels prepared with 4% BW suggests a greater degree of self-sorting and rearrangement of the beeswax, resulting in the formation of more crystals, as reported by Zhao et al. [13].
3.3.5. Rheological and Textural Properties
A frequency sweep test was performed to assess the elastic modulus (G′), viscous modulus (G″), and complex shear modulus (G*)/frequency of all the oleogels (Fig. 2). Overall, G′ was greater than G″ for all oleogels, and both moduli increased slightly but gradually across the entire frequency range. These results indicate that the oleogels produced with beeswax (BW) were viscoelastic semi-solids with good tolerance to deformation.
As observed, oleogels with higher G′, G″, and G*/ frequency values were typically obtained as the wax concentration increased. Similarly, increasing the CLA ratio at the same oleogelator concentrations also led to higher G′, G″, and G*/frequency values. It has been reported that an increase in G* is associated with enhanced gel strength [2, 15]. In the present study, it was found that G*/frequency increased as the ratios of CLA and BW increased, indicating that the gel strength of the oleogel was enhanced with higher concentrations of BW and CLA. The ratio G*/frequency represents the viscosity as a function of frequency, analogous to the relationship between viscosity and shear rate. These findings were also supported by the firmness results.
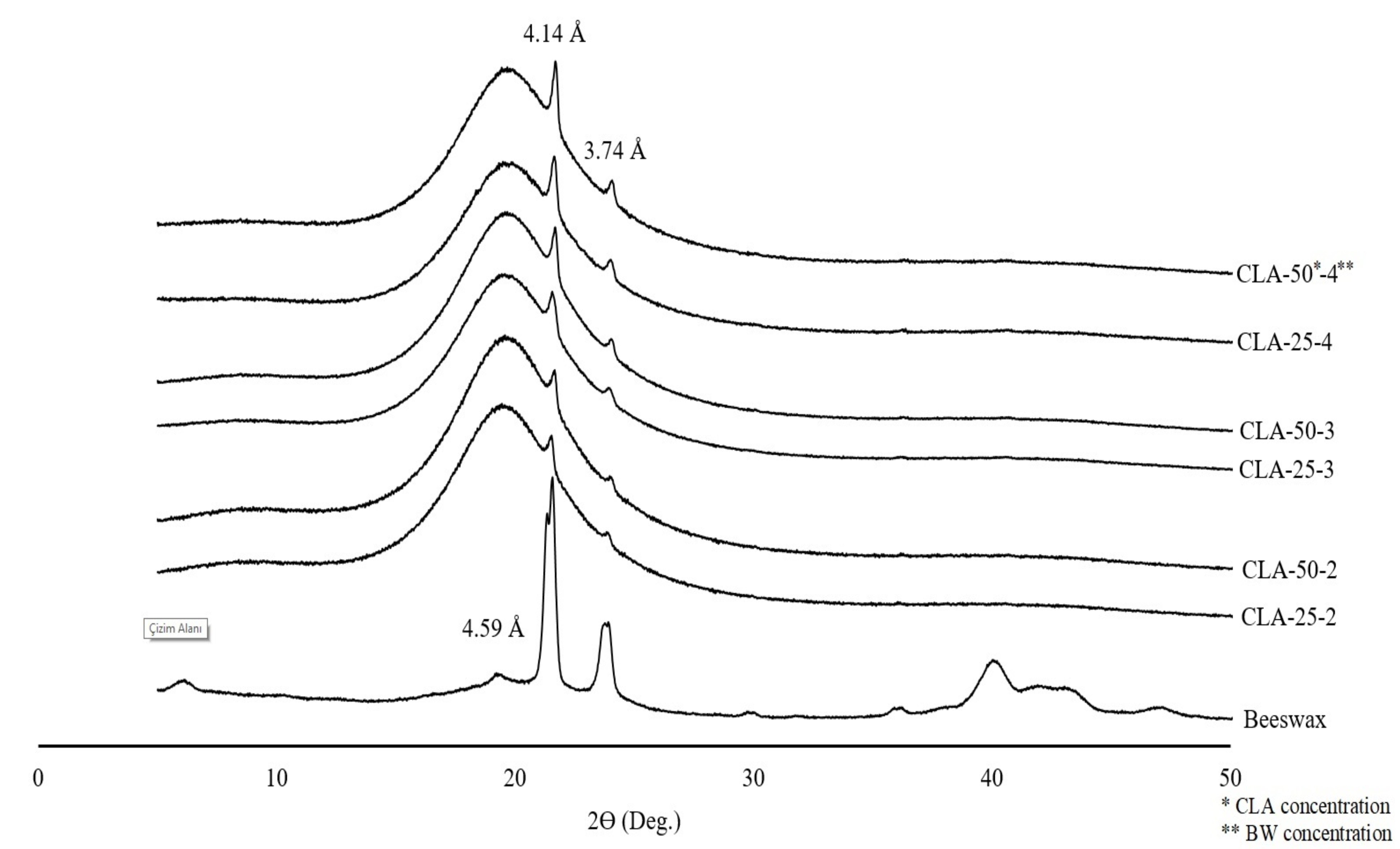
The X-ray diffraction patterns of the oleogels and BW.
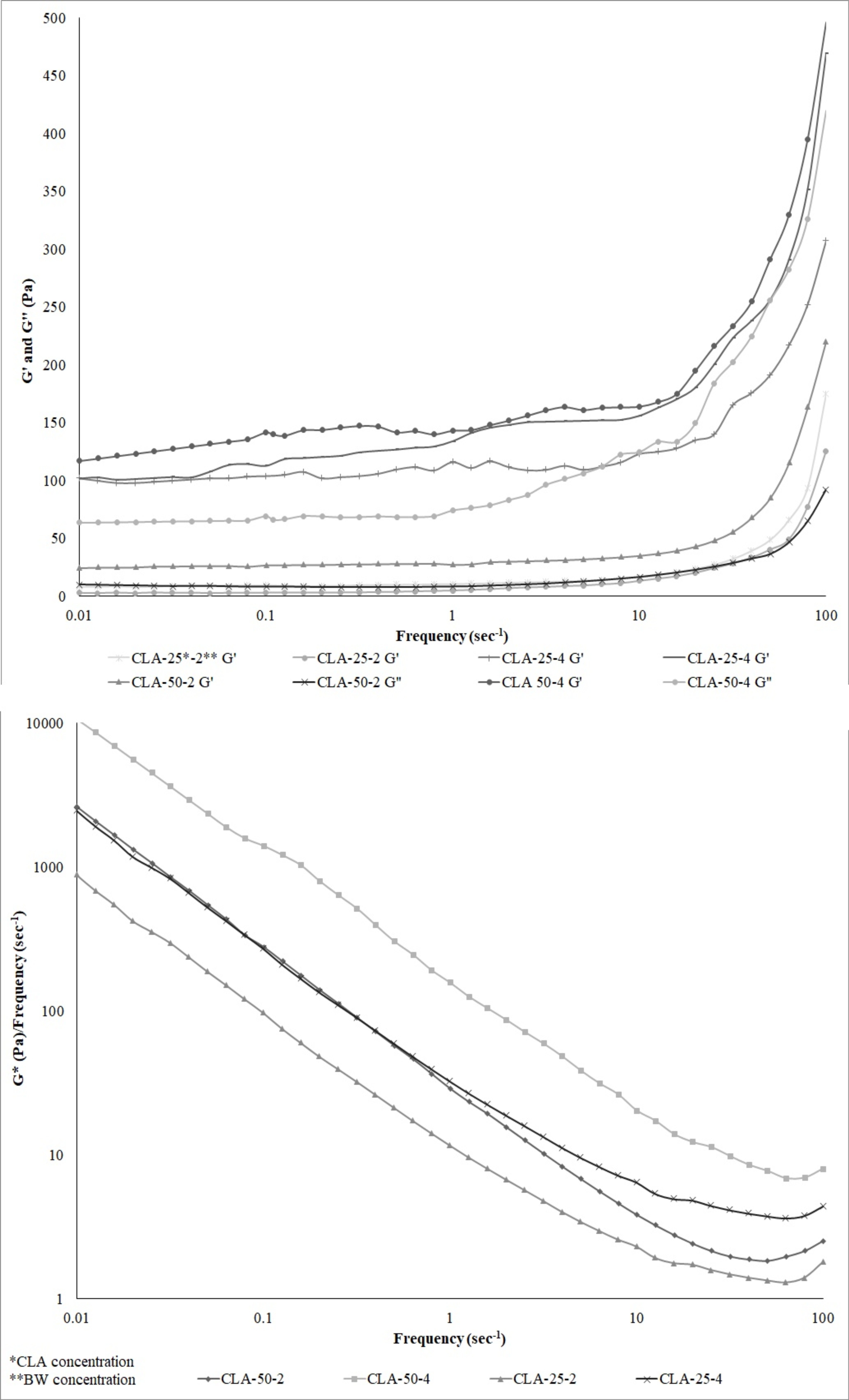
Frequency dependence of the elastic modulus (G´), viscous modulus (G´´) and complex shear modulus (G*)/frequency for oleogels at 25°C.
This effect can be attributed to the higher melting points of CLA isomers (19.8°C for 10t-12c CLA and 14.9°C for 9c-11t CLA) compared to LA (−5.2°C) and oleic acid (13.3°C) [16]. Consequently, the same concentration of gelator formed stronger and more robust gel networks with increasing CLA content. This finding supports the hypothesis that CLA exerts a synergistic effect in oleogel formation. Consistent with this hypothesis, higher CLA concentrations resulted in firmer gels with more stable structures. The firmness of the oleogels, a key indicator of gel strength, further corroborated these observations (Fig. 3).
The formation of a stable oleogel requires a higher concentration of gelator. In this context, CLA not only contributed to the effective gelator content but also enhanced the viscoelastic properties of the oleogels. The incorporation of CLA resulted in increased firmness, exceeding that of the control samples. Notably, the oleogel containing 50% CLA and 4% BW exhibited the highest firmness among all formulations. It is generally accepted that crystal structures with higher G′ values tend to form a larger number of smaller crystals [13], which may account for the structural characteristics observed in the CLA-50-4 samples.
3.4. Storage Stability of Oleogels
For oxidative stability analyses, sunflower oil (Control-0) and CLA-enriched oils without added beeswax (Control-25 and Control-50) were used as control samples. The PV (meq O2/kg) and TBA (mg MDA/kg) values of the samples are presented in Fig. (4). The effects of BW and CLA concentrations on PV were not statistically significant during the first 15 days of storage (p > 0.05). However, after 15 days, the PVs of all samples increased significantly with prolonged storage at 25°C (p < 0.05). Despite this increase, PV values remained below 10 meq O2/kg throughout the 120-day storage period. In contrast, the TBA values decreased with increasing BW concentration. Notably, although all control samples exhibited similar TBA values up to day 30, samples containing CLA (Control-25 and Control-50) showed lower TBA values than Control-0 after day 30, indicating the antioxidant effect of CLA. However, the higher TBA value observed in samples with 50% CLA compared to those with 25% CLA suggests that excessive CLA may reduce oxidative resistance (Fig. 4).
Although the concentration of oleogelators has been reported to influence peroxide formation during storage [2], some studies have indicated that the concentration of waxes such as sunflower, BW, and carnauba does not significantly affect lipid oxidation. Conversely, it is generally accepted that CLA oxidizes more rapidly than LA [16]. However, Yettella et al. [17] observed a substantial increase in the PV of soybean oil during storage, while no significant change was detected in the PV of CLA-containing samples. This finding aligns with the report that hydroperoxides are not the primary oxidation products of CLA [18]. Furthermore, it has been suggested that the combination of CLA and LA may exhibit a prooxidant effect under oxygen exposure and that the antioxidant or prooxidant behavior of CLA may depend on its specific isomeric form [19].
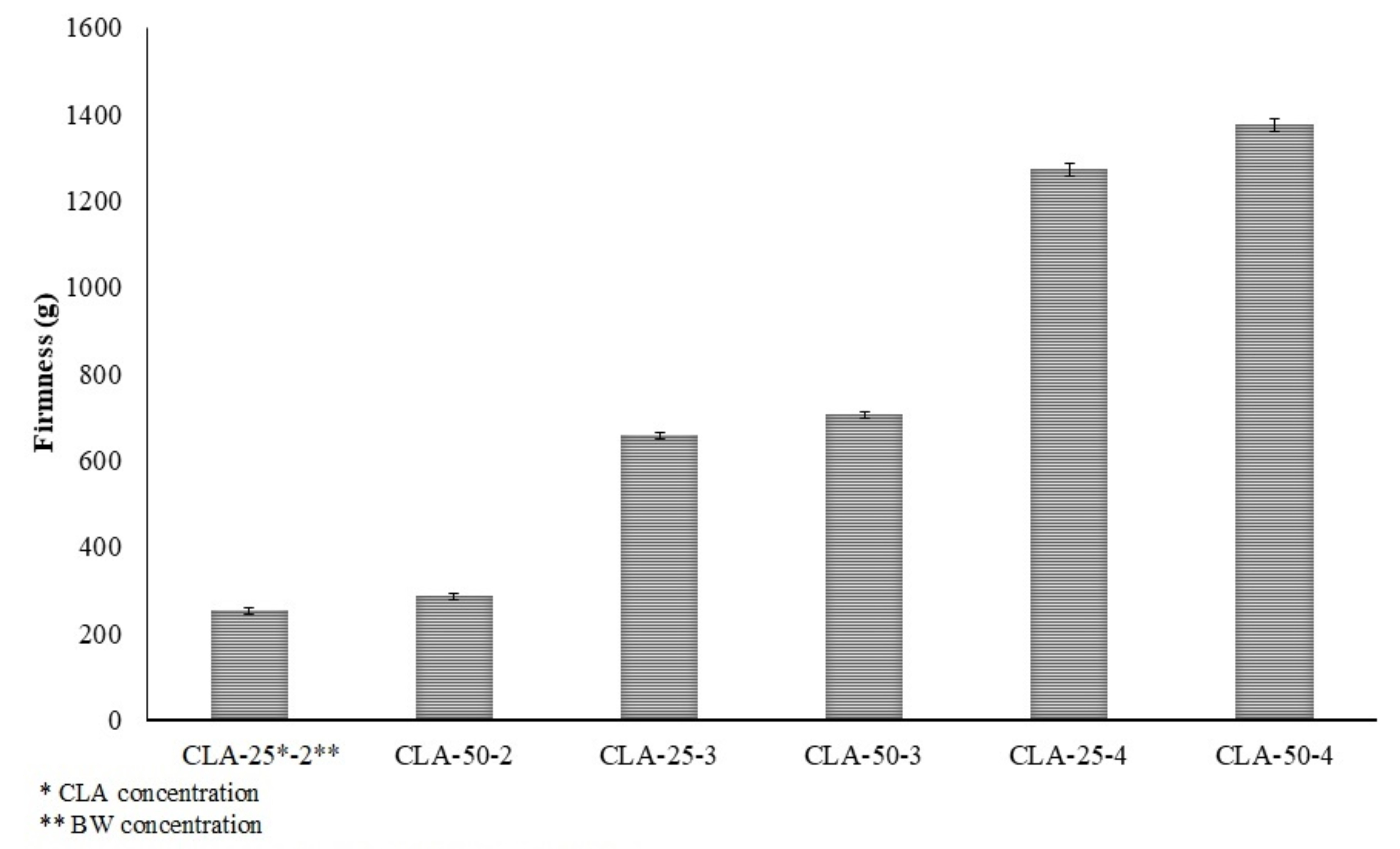
Firmness values of oleogels at 25°C.
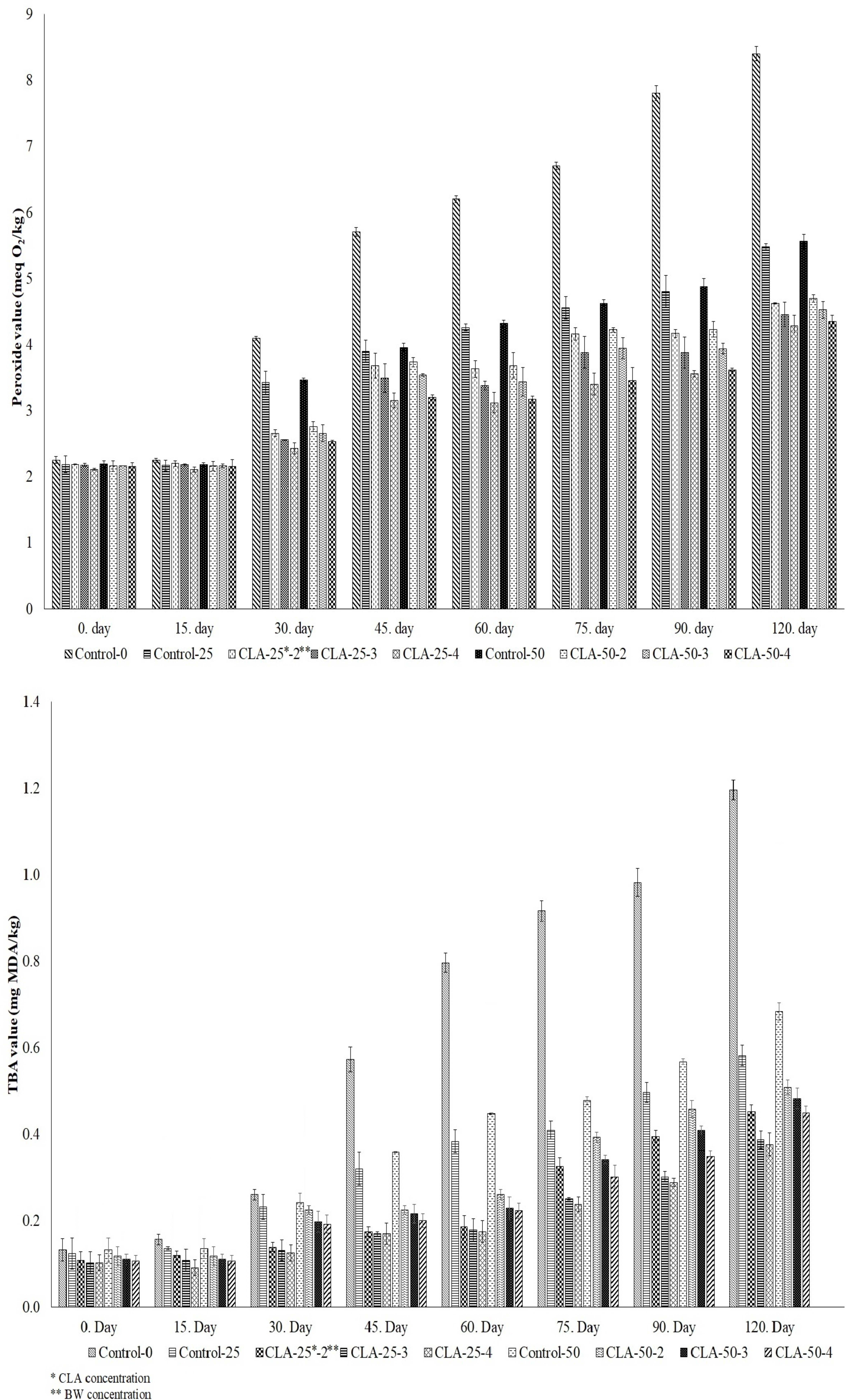
Peroxide (PV) and TBA values of oleogel samples during 120 days.
“In this study, all oleogel samples exhibited lower PV and TBA values compared to the control samples throughout the 120-day storage period. These findings suggest that increasing the concentration of BW or CLA (specifically 9c-11t and 10t-12c isomers) reduces the susceptibility of the oil to oxidative degradation. Similar results have been reported by Okur and Erinç [2], Oh et al. [20], and Li et al. [21].
Considering all these findings, the synergy between CLA and BW appears to stem from molecular-level interactions that influence both crystal structure formation and oxidative stability within the oleogel matrix. The relatively high melting point of CLA, coupled with its conjugated double-bond structure, may allow it to integrate into the beeswax crystalline network, thereby modifying the size, morphology, and density of the resulting crystals. This structural alteration appears to enhance both the firmness and oil-binding capacity of the gel. However, elevated CLA concentrations may disrupt the regularity of the crystal lattice, potentially leading to decreased thermal stability.
On the other hand, the antioxidant properties of CLA significantly enhanced the long-term oxidative stability of the system by inhibiting lipid oxidation. Throughout storage, CLA’s free radical scavenging capacity effectively delayed oxidative degradation. In this context, the oleogel structure, formed with CLA and BW, exhibited improved oxidative resistance despite a minor reduction in thermal stability. These findings underscore a well-balanced synergy between the structural integrity and functional performance of the oleogel, highlighting the dual role of CLA in enhancing both the physical and oxidative properties of the system.
CONCLUSION
In this study, the production of oleogels containing BW and CLA was demonstrated for the first time. The structural properties of the oleogels containing CLA were systematically characterized and compared with the control samples. Furthermore, the oxidative changes of the oleogels were monitored over 120 days.
Overall, BW was found to be an effective gelator for sunflower oil oleogels containing 25% and 50% CLA at a minimum gelator concentration of 2 wt%. According to textural and rheological analyses, BW forms an elastic gel upon crystallization, making it a promising gelator. However, the spreadability of the oleogels increased with higher CLA content, while it became more challenging with increasing BW concentrations.
As the CLA concentration increased from 25% to 50%, the oleogels exhibited greater yellowness. However, CLA had no significant impact on brightness or greenness. The thermo-oxidative stability of the oleogels decreased with the addition of both BW and CLA, with the oxidative stability improving due to CLA’s resistance to oxidation, despite the introduction of less stable wax.
Moreover, as the BW and CLA concentrations increased in the oleogel formulation, the values of G′, G″, and firmness also increased. Consequently, increasing CLA produced a firmer and more stable gel structure. This suggests that CLA-containing oleogels are unstable at high temperatures (>100°C) but stable at lower temperatures (25°C). It is important to note, however, that thermal and oxidative stability decreased as the CLA concentration increased from 25% to 50%.
The beneficial effects of incorporating CLA into the oleogel formulation were evidenced by its improved storage stability, enhanced antioxidant properties, better textural structure compared to control samples, and positive results in the characterization analyses. The use of CLA in food products warrants further investigation due to its potential positive effects on the final product and its known health benefits.
STUDY LIMITATIONS
This study has certain limitations, including the exclusive use of sunflower oil and the assessment of storage stability at a constant temperature of 25°C. Additionally, sensory analysis and food application evaluations were not conducted. Future research should focus on examining the behavior of oleogels under cooking conditions to better assess their practical applicability in food formulations.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contributions to the manuscript as follows: H.E.: was responsible for study conception and design, while; O.U.T.: Conducted the data collection;. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| CLAs | = Conjugated Linoleic Acids |
| FDA | = Food and Drug Administration |
| FID | = Flame Ionization Detector |
AVAILABILITY OF DATA AND MATERIAL
All the data and supporting information is provided within the article.
FUNDING
This work was supported by the Scientific Research Projects Unit of Niğde Ömer Halisdemir University, Turkey (Project No: GTB 2018/15-HIDEP).
ACKNOWLEDGEMENTS
Declared none.


