All published articles of this journal are available on ScienceDirect.
Effect of Boron Doping on Sensing Properties of CNTs Functionalized with Nitro Group
Abstract
Introduction
To date, there has been no systematic study of the effect of substituting boron atoms on various types of sensory interaction.
Methods
In order to clarify the mechanisms of sensory interaction of boron-carbon nanotubes with respect to metal atoms and to establish the effect of modification by a nitro group on them, the results of model experiments conducted using density functional theory (DFT) were used. The mechanism of functionalization is presented in this work. The sorption and sensing interaction of the obtained nanosystems with alkali metal atoms (Li, Na, K) were evaluated to assess the efficiency of each of the nanosystems considered in this work.
Results
The influence of impurity boron atoms replacing the carbon atoms of the nanotube surface on the sensory properties of the CNTs was determined.
Discussion
The energetically favorable and preferable location of the nitro group for this process is above the surface boron atom for all boron-carbon nanotubes considered. The conductivity of such systems changes upon interaction with alkali metal atoms, which makes it possible to register their presence.
Conclusion
The results of the study of the mechanisms of sensor interaction between alkali metal atoms and boron-carbon nanotubes functionalized with a nitro group, containing different amounts of impurity boron atoms replacing carbon atoms of the carbon nanotube surface, allow us to conclude that the obtained systems are able to register the presence of selected metal atoms (Li, Na, K).
1. INTRODUCTION
In the sensor industry, there has recently been a tendency to create sensors that do not contain metal particles, since they allow for high sensitivity and selectivity when used as the main carbon material. Hybrid composite nanosensors can achieve a long lifetime along with a low cost of the device, as well as controlled electronic structure, stability, high sorption properties, rapid electron transfer, and biocompatibility [1].
Carbon nanotubes are a new class of one-dimensional materials used as electrodes in sensor devices, due to their high specific surface area, conductive and sorption properties, chemical stability, and biocompatibility [2, 3]. However, this class of nanomaterials is not without its drawbacks, among which the most significant is homogeneous electron density, which reacts unsatisfactorily to sensory interactions [4]. In the course of the experiments, it was found that boron doping not only creates charge redistribution, but also leads to the possibility of sensitivity control by changing the concentration of the alloying element [3-5].
Boron is one of the most likely candidates for the modification of carbon nanotubes due to several factors. First of all, this is due to the fact that the substitution reaction does not significantly disrupt the surface structure and symmetry of the nanotubes [5]. Due to the lack of electrons compared to carbon, boron is widely used as a doping material for carbon nanotubes not only in sensors but also in other industries, for example [6].
As noted by the authors, the resulting electronic heterogeneity due to the appearance of heteroatoms leads to an improvement in sorption, sensory, and electrochemical interactions [7-10].
Often, the study of a particular type of nanomaterial looks impractical, since the technology for its production has not yet been established. However, carbon nanotubes modified with boron have been produced for more than ten years [5, 11-13]. The first publications devoted to the study of their sensory properties in relation to various elements also date back to this time [14-16]. Currently, various nanostructures, including nanotubes, have been modified with boron and successfully tested as sensors [17-21].
However, to date, there has been no systematic study of the effect of substituting boron atoms on various types of sensory interaction. The formation of such a systematic approach will make it possible in the future to unambiguously determine the level of introduction of replacement materials to achieve a specific effect. For this purpose, the study described in this paper was conducted.
The aim of this work is to computationally investigate the mechanisms of functionalization of the surface of CNTs modified by boron atoms with different impurity concentrations (50%, 25%, and 15%) by studying the process of nitro group adsorption on the surface of such boron-carbon nanotubes. The sensory activity of such functionalized nanosystems with atoms of alkali metals, such as lithium (Li), sodium (Na), and potassium (K), which are potential metal pollutants of the environment and are part of various salts, is also investigated.
2. METHODOLOGY
According to density functional theory (DFT), the properties of a multi-electron system, including energy, can be determined using the electron density functional. The system is described by the electron density as ρ(r) according to Eq. (1):
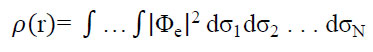 |
(1) |
where Φe is the many-electron wave function of the system, σi is the set of spin and spatial coordinates of the electrons, and N is the number of electrons.
Thus, ρ(r) is a function of only three spatial coordinates r of the point at which ρ(r) gives the probability of detecting any of the electrons in the molecule [22].
If any property of the ground state of a molecule can be expressed as a function of ρ, then the electron energy in DFT is of the form presented in Eq. (2):
| E[ρ] = T[ρ] + Ven[ρ] + Vee[ρ] | (2) |
where T[ρ] is the kinetic energy, Ven[ρ] is the potential energy of electron-nuclear interactions, and Vee[ρ] is the energy of electron-electron interactions, which can be written in the form of Eq. (3):
| Vee[ρ] = VCoul[ρ] + Vxc[ρ], | (3) |
where VCoul[ρ] is the Coulomb interaction energy of electrons and Vxc[ρ] is the exchange-correlation energy.
The functionals T[ρ], Ven[ρ] and VCoul[ρ] can be precisely determined [23]. For the exchange-correlation potential Vxc[ρ] the exact representation is unknown, and there are a large number of models to describe it.
B3LYP is one of the most popular and widely used functionals in DFT calculations. It combines local density approximation (LDA) and gradient exchange in the form of generalized gradient approximation (GGA) with some contributions from Hart-Fock (HF) parameters, which makes it suitable for the description of various types of molecules and chemical reactions. The B3LYP functional was first proposed by Becke in 1993 [24] and quickly became a popular choice for a wide range of applications including organic, inorganic, and photochemical systems. The functional is also used to determine energies, molecular geometries, electronic excitation spectra, thermochemical parameters, reaction barriers, and more. It is based on a hybridization method that combines local functionals and the Hartree-Fock functional, with correlation energy applied in some proportion.
The main benefits are:
- Applicability to a wide range of systems, from small organic molecules to biologically active compounds and transition metals.
- Better accuracy than other functionals, while requiring fewer computational resources than, for example, high-level approximation methods such as CCSD(T).
- The possibility to describe both covalent and ionic bonds.
Some disadvantages of B3LYP, however, must be considered:
- The functional may be inaccurate for some systems, such as those containing transition metals or strongly polarizable atoms.
- It is not always possible to correctly describe systems with very long bonds using this functional because of insufficient correlation energy.
- B3LYP may give an incorrect description of donor-acceptor bonds, especially in systems with a large charge distribution.
Another feature of this functional is that the three exchange components are fitted with coefficients chosen on the basis of comparison with experimental data. As a result, the functional acquires the features of a semi-empirical method. It turns out that its accuracy in most cases is significantly higher than that in the case of methodologically “pure” functionals. Apparently, this is a consequence of the fact that the exchange energy has a nonlocal character, and any attempts to reduce it to local functionals lead to errors. The Hartree-Fock exchange allows one to take into account this nonlocality.
In general, B3LYP is a well-balanced functional for most chemical applications. It has sufficient accuracy and versatility to be useful for the description of various molecular systems, including those considered in the present work. Therefore, in the presented theoretical study, the B3LYP functional was used in the framework of density functional theory.
The 6-31G valence-splitting basis is one of the most widely used bases in quantum-chemical calculations. It was developed for use in calculations of organic molecules, which contain elements from the first to the seventh group of the periodic table [24]. The valence-split basis means that the basis functions in this set are divided into two groups: valence and split functions. The valence functions are used to describe the electronic regions of the outer electron shells of the molecule, while the split functions describe the behavior of electrons inside the atomic nuclei. This separation allows for a more accurate account of the electrostatic interaction between electrons and nuclei.
The number 6 in the name of the 6-31G basis indicates the number of basis functions for each atom in the system. In this case, 6 basis functions are used for hydrogen and halogens (the first and seventh groups), and 31 functions are used for all other elements. The number of basis functions in 6-31G provides sufficiently high accuracy of calculations, while maintaining an acceptable calculation time. Other existing bases, for example, 3-21G and STO-3G, in turn, contain fewer functions and provide faster calculations, but their accuracy is usually insufficient for the description of complex organic systems.
Computer experiments were performed using templates in the ORCAD software package, which is distributed free of charge.
The choice of functional and basis set in this study was not arbitrary. As noted above, they take into account the electronic structure of all the elements involved in this experiment. Additionally, the authors conducted a comparative analysis between the results of computational modeling and experimental data on boron–carbon nanostructures. As a representative example, below is a table with a comparison of the band gap values obtained by us and other researchers for boron-carbon nanotubes. To compare the results, Table 1 shows the values of the band gap width obtained as part of the experiment and the computer model presented.
As follows from the table, the values are close to each other, so we can conclude that the models used in this study are valid. A number of researchers have also conducted studies of the sensory properties of nanocarbon materials in the same computational framework, as discussed in more detail [27-29].
Therefore, we can consider that the choice of the 6-31G basis in quantum-chemical calculations is justified by its ability to describe nanosystems quite accurately, while providing a moderate calculation time.
An equilibrium concentration of boron and carbon atoms in the nanotube structure, corresponding to 50% atomic substitution, referred to as BC nanotubes, was selected as the initial model for investigating the influence of substituted boron atoms on the sensory properties of carbon nanotubes. Nanotubes with a zigzag configuration were examined in this study. The spatial arrangement of boron and carbon atoms is presented in Fig. (1).
Variant atomic ordering of B and C atoms in the BC cluster of a (6,0)-type nanotube.
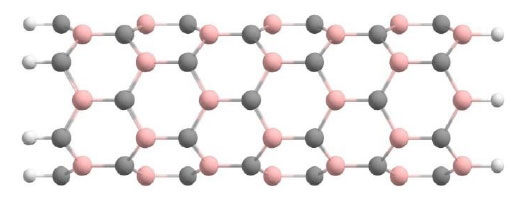
Then we modeled nanotubes in which the content of substituted boron atoms was up to 25%. We recognized these nanostructures as BC3 nanotubes. Fig. (2) shows a fragment of a cluster of the considered type of nanotubes.
Variant atomic ordering of B and C atoms in the BC3 cluster of a (6,0) type nanotube.
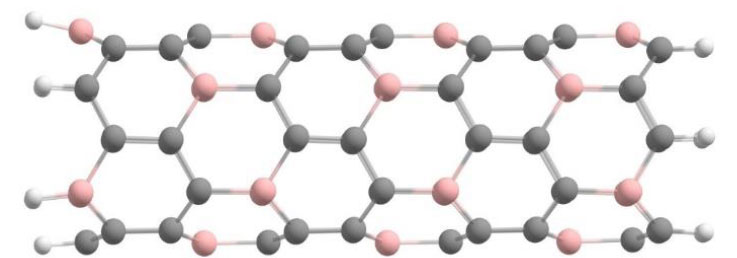
Next, we studied nanotubes in which only one carbon atom in the hexagon is replaced by a boron atom, i.e., the structure of the BC5 type containing 15% of boron atoms. This structure corresponds to the minimum concentration of boron atoms that ensures a uniform distribution of it throughout the volume of the nanotube. A fragment of a BC5 nanotube with a zigzag configuration is shown in Fig. (3).
Variant atomic ordering of B and C atoms in the BC5 cluster of a (6,0) type nanotube.
Thus, three models of nanotubes containing periodically distributed substituted boron atoms on their surface were constructed. The length of the simulated cluster for each case was 22.4 Å. The diameter of the nanotubes was 4.77 Å. Hydrogen atoms were used as pseudoatoms to compensate for the broken bonds at the cluster boundary. The model has been well tested for studying the processes of sensory and sorption interaction. It does not require a lot of machine time, but at the same time accurately reproduces point processes on the surface of a nanotube, which include cases of sensory interaction.
3. RESULTS
Carbon nanotubes are extensively studied, there are many publications on this subject. However, if we return to doped carbon nanotubes, it is boron-doped CNTs that are relatively understudied. The main focus of publications has been on the synthesis of boron-doped carbon nanotubes using various precursors. Few publications are devoted to the use of these boron-carbon nanotubes as sensors for sensing devices such as in our work, with the existing works being primarily theoretical in nature.
Yao and his coworkers have extensively studied the experimental application of boron-carbon CNTs in biosensors [14, 30-32]. In [14], the authors reported a boron-carbon CNTs modified glassy carbon electrode for the detection of glucose (as an amperometric biosensors). This electrode was prepared by entrapping glucose oxidase enzyme into poly(o-aminophenol) film. In article [30], the authors also reported the use of boron-carbon nanotubes modified glass carbon electrodes for detecting glucose. In this case, the glucose oxidase enzyme was immobilized on a BCNTs modified glassy carbon electrode.
The response of pristine, nitrogen and boron doped carbon nanotube (CNT) sensors to NO2, CO, C2H4 and H2O at ppm concentrations was investigated at both room temperature and 150 °C [13]. B-doped CNTs showed the best sensitivity to ethylene. All tubes (including undoped) showed strong humidity response. Sensing mechanisms were determined via comparison with density functional calculations of gas molecule absorption onto representative defect structures in N and B-doped graphene. B-CNTs appear to react chemically with many of the absorbed species as shown by their poor baseline recovery and increasing sensitivity with temperature. This limits their cyclability. Overall gas sensitivity is as good as or better than that of post-growth functionalised nanotubes, and used in combination, CNTs, N-CNTs and B-CNTs appear to be highly promising candidates for cheap, low power, room temperature gas sensing applications.
These experimental works indicate the interest of the scientific community in exploring similar carbon nanomaterials as sensors for sensing devices. In addition, such nanomaterials are actively used in medicine, but are seldom used for protection against harmful gases and poisonous substances.
To improve the sensory properties of BC, BC3, and BC5 nanotubes, their surfaces were functionalized with a nitro group (-NO2), the presence of which can lead to an increase in the sensitivity of nanotubes to various atoms and molecules.
Surface functionalization of boron-carbon nanotubes [26, 33-36] was carried out by attaching a nitro group to two possible nanotube surface centers in 0.1 Å increments: (1) - surface carbon atom; (2) - surface boron atom. The functional group was placed perpendicular to the nanotube axis and approximately in the center of the cluster to eliminate the influence of edge atoms. Molecular clusters of boron-carbon nanotubes of type (6,0) contained six six-atom cycles (hexagons) along the longitudinal axis of the nanotube, and the broken bonds at the cluster boundaries were terminated with pseudo-hydrogen atoms. As an example, Fig. (4) shows a model of a BC5 boron-carbon nanotube cluster with a nitro group placed on its surface. This figure illustrates a typical algorithm for constructing a model for the interaction between a functional group and a nanotube. The nitrogen atom, highlighted in blue, was used to bring the functional group closer to the nanotube, while the oxygen atoms are shown in red. Fig. (4a) shows a cross-sectional view of the model used in the computer experiment, where it can be seen that the nitro group is attached perpendicular to the surface of the nanotube. The approximation was made by
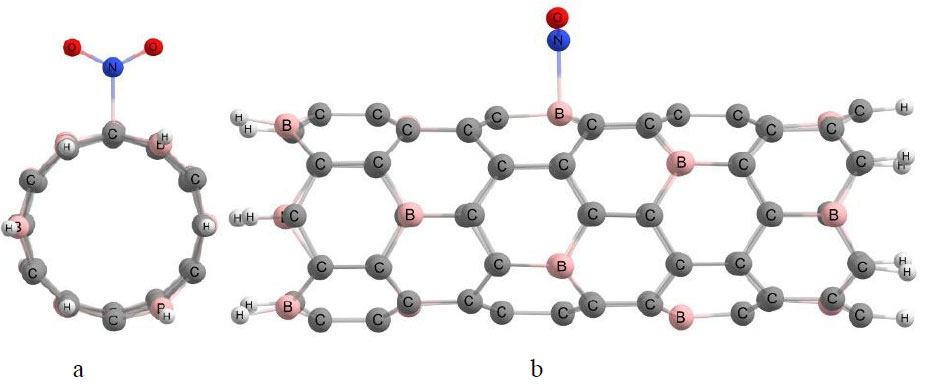
Model of boron-carbon BC5 nanotube with -NO2 nitro group attached to its surface: (a) Frontal view; (b) Side view.
positioning the nitrogen atom near a surface atom of the nanotube. Fig. (4b) shows a longitudinal view of the computer model, demonstrating that the functional group is located at the center of the cluster.
The results of nitro group attachment to the surface of boron-carbon nanotubes are shown in Table 2 and as energy curves in Fig. (5). The nitro group was attached to carbon nanotubes with varying concentrations of substituted boron atoms. Fig. (5a) shows the energy curves for nanotubes containing boron and carbon in equal parts. Fig. (5b) presents the potential energy surface profiles for nanotubes containing 25% substituted boron atoms. Fig. (5c) shows the energy curves for the minimum boron concentration under consideration, namely 15%.
4. DISCUSSION
Based on the analysis of the obtained data, the following conclusions can be drawn regarding the mechanism of functionalization of the nanotube surface by a nitro group:
- Functionalization of BC and BC3 nanotubes surface is possible through the attachment of a nitro group to either a carbon atom or boron atom. In contrast, for BC5 nanotubes, functionalization is only feasible when (-NO2) is attached to a boron atom on the tube surface. Attachment of the nitro group to a carbon atom is not possible, and the interaction energy of the group with the nanotube takes a positive value. These conclusions are based on the presence of minima on the energy curves. The distance at which the minimum occurs corresponds to chemical adsorption, indicating the formation of a stable nanotube-functional group complex, which may subsequently act as an element of a sensor;
- Analysis of the charge state of the systems revealed that in all cases there is a transfer of electron density from the nitro group to the nanotube, which leads to the appearance of additional charge carriers in the systems and affects their conductivity;
- The analysis of the energy gap width (Egap) of the complexes showed that it increases with increasing concentration of impurity boron atoms in functionalized nanosystems.
Fig. (6) shows the density of states (DOS) plots illustrating the contribution of the nitro group to the structure of boron-carbon nanotubes. Fig. (6a) shows the DOS of a nanotube containing 50% carbon and boron atoms with an attached nitro group as it approaches a carbon atom. The DOS for a similar nanotube in the case of an approximation to a boron atom is shown in Fig. (6b). Next, the densities of states for nanotubes containing 25% substituted boron atoms were constructed. Fig. (6c) shows the DOS in the case of approximation to carbon, in Fig. (6d) - to boron. For a nanotube with a minimum percentage of substituted boron atoms, adsorption is realized only when approaching a boron atom, the density of states for this case is shown in Fig. (6e). As can be seen in the graphs, each time the interaction with the nitro group leads to a change in the energy structure of the complex. This can be explained by the fact that approaching atoms with different electronegativity changes the levels of molecular orbitals, although the value of the band gap itself practically does not change depending on the atom to which the approximation is implemented. However, the electronic distribution resulting from the introduction of heteroatoms leads to the appearance of additional charge carriers, which positively affects the possibility of using sensors according to the principles described above.
|
Type of Nanotubes |
Adsorption Center |
r, Å | Eint, eV | Egap, eV | Charge |
|---|---|---|---|---|---|
| BC | (a) carbon atom | 1.6 | -1.11 | 0.85 | 0.31 (N), -0.27 (O) |
| (b) boron atom | 1.8 | -0.36 | 0.85 | 0.25 (N), -0.28 (O) | |
| BC3 | (a) carbon atom | 1.9 | -0.13 | 0.6 | 0.42 (N), -0.27 (O) |
| (b) boron atom | 1.9 | -0.17 | 0.55 | 0.28 (N), -0.3 (O) | |
| BC5 | (a) carbon atom | 2.0 | 0.53 | 0.47 | 0.46 (N) / -0.27 (O) |
| (b) boron atom | 1.8 | -0.2 | 0.32 | 0.23 (N), -0.31 (O) |
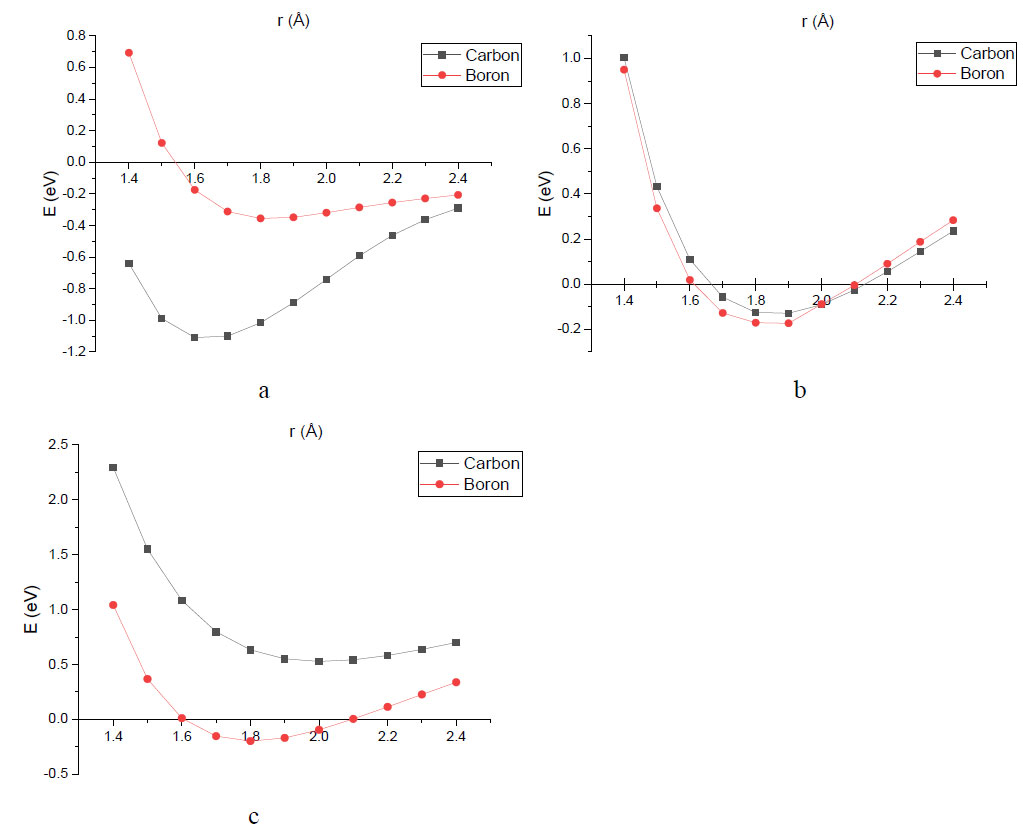
Energy curves characterizing the adsorption process of nitro group on the nanotube surface: (a) to BC nanotube; (b) to BC3 nanotube; (c) to BC5 nanotube.
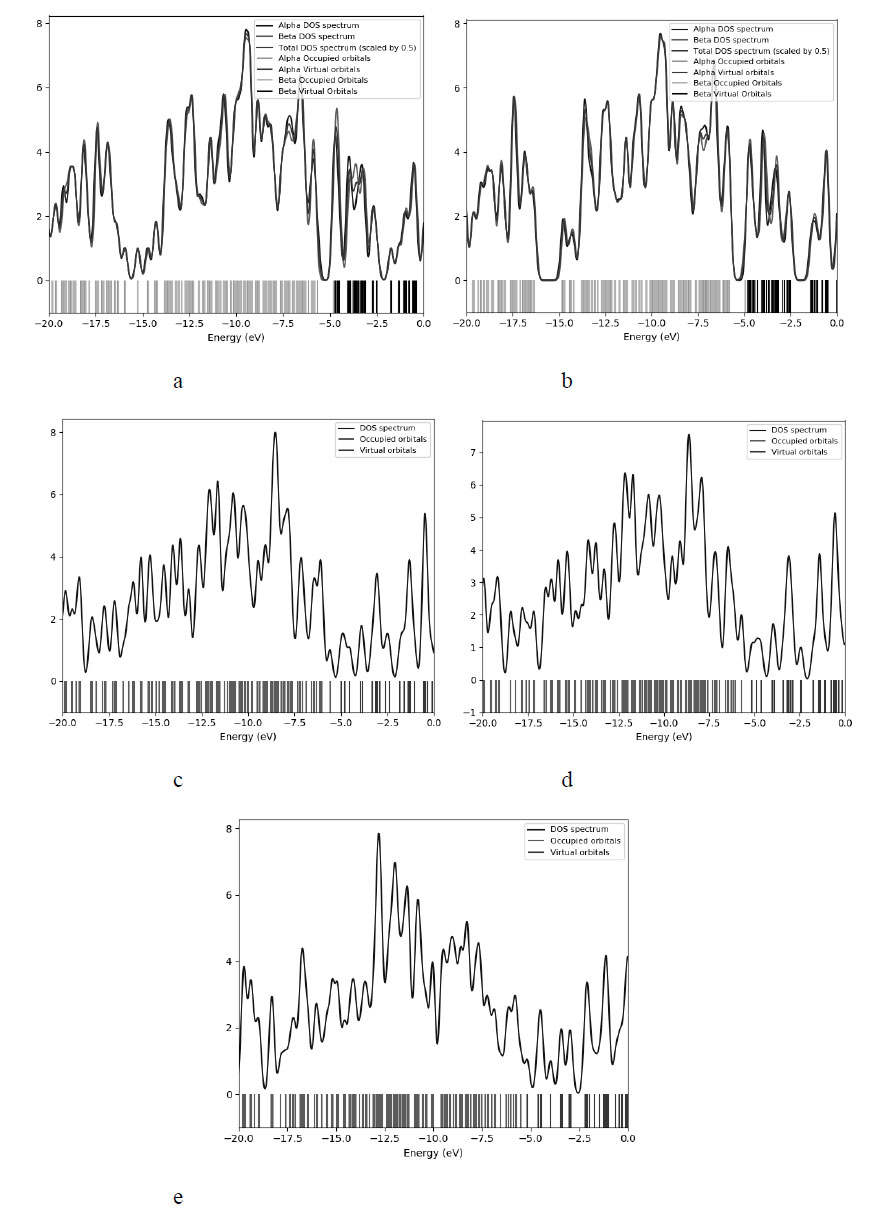
Density of states (DOS) plots illustrating the process of functionalization by the nitro group - NO2 of boron-carbon nanotubes: (a) NO2 at a distance of 1.8 Å above the carbon atom of the BC nanotube surface; (b) NO2 at a distance of 1.8 Å above the surface boron atom of the BC nanotube; (c) NO2 at a distance of 1.9 Å above the surface carbon atom of BC3 nanotube; (d) NO2 at a distance of 1.9 Å above the surface boron atom of BC3 nanotube; (e) NO2 at a distance of 1.8 Å above the surface boron atom of BC5 nanotube.
To determine the distance and energy of sorption interactions in the obtained functionalized complexes, the approximation of alkali metal atoms (lithium, sodium, potassium) to one of the oxygen atoms of the functional group with a step size equal to 0.1 Å was simulated. As a result, the energy curves of the process were constructed (Fig. 7) and the main parameters of the interaction were determined, as shown in Table 3. For a better understanding, the profiles of potential energy surfaces were grouped by metal atoms. Fig. (7a) shows the energy curves of the interaction of lithium atoms with a nanosystem. Similar graphs for the sodium atom are shown in Fig. (7b). Fig. (7c) shows potential energy surface profiles for the interaction of various types of nanotubes with potassium atoms.
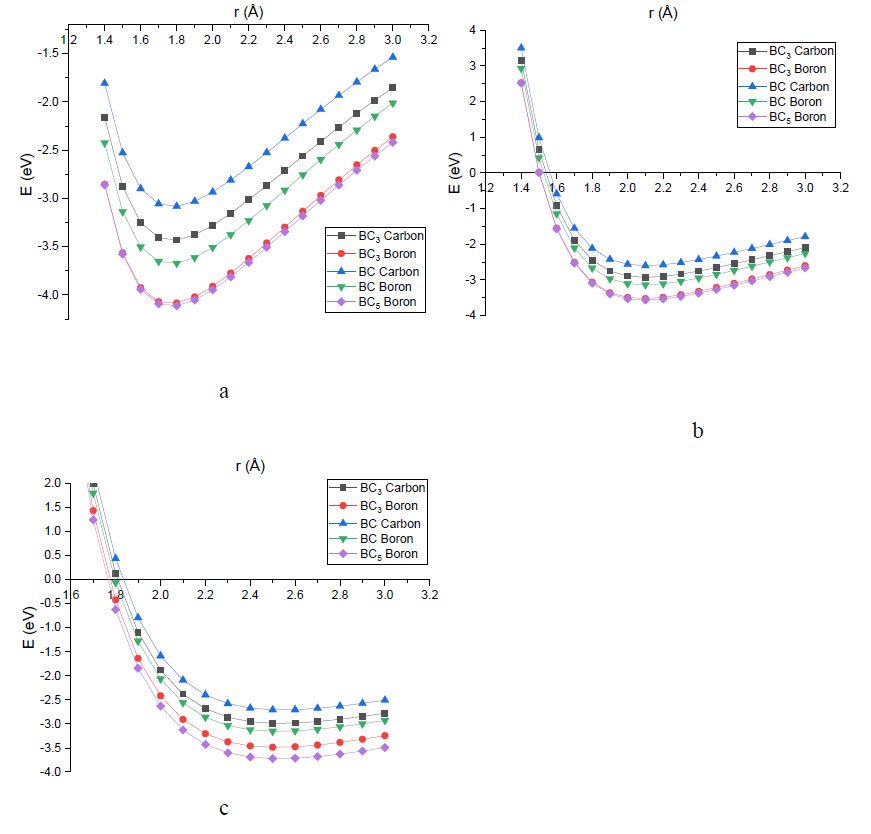
Energy curves characterizing the process of sorption interaction of modified nanotubes with atoms of: (a) lithium; (b) sodium; (c) potassium.
|
Type of Nanotubes |
Adsorption Center |
r, Å | Eint, eV | Egap, eV | Charge |
|---|---|---|---|---|---|
| Li | |||||
| BC | (a) carbon atom | 1.8 | -3.08 | 0.66 | 0.63 |
| (b) boron atom | 1.8 | -3.67 | 0.95 | 0.6 | |
| BC3 | (a) carbon atom | 1.8 | -3.43 | 0.71 | 0.62 |
| (b) boron atom | 1.8 | -4.09 | 0.7 | 0.58 | |
| BC5 | (a) boron atom | 1.8 | -4.11 | 0.87 | 0.6 |
| Na | |||||
| BC | (a) carbon atom | 2.1 | -2.6 | 0.67 | 0.66 |
| (b) boron atom | 2.1 | -3.14 | 0.94 | 0.7 | |
| BC3 | (a) carbon atom | 2.1 | -2.93 | 0.71 | 0.63 |
| (b) boron atom | 2.1 | -3.52 | 0.7 | 0.64 | |
| BC5 | (b) boron atom | 2.1 | -3.56 | 0.87 | 0.66 |
| K | |||||
| BC | (a) carbon atom | 2.5 | -2.71 | 0.66 | 0.78 |
| (b) boron atom | 2.5 | -3.15 | 0.93 | 0.75 | |
| BC3 | (a) carbon atom | 2.5 | -2.99 | 0.75 | 0.67 |
| (b) boron atom | 2.5 | -3.49 | 0.7 | 0.74 | |
| BC5 | (c) boron atom | 2.5 | -3.72 | 0.88 | 0.76 |
The analysis of the obtained results showed that the interaction of the selected metal atoms with the oxygen atom of the group is barrierless (Fig. 7). The sensor using boron-carbon nanotubes functionalized with a nitro group as an active material is able to register the change in the Schottky barrier between the electrodes of the sensor device and functionalized nanosystems, which is illustrated by the change in the potential in the system when the interaction energy changes. An increase in the number of impurity boron atoms in the structure of carbon nanotubes leads to an increase in the interaction energy of nanosystems with alkali metal atoms.
After the distances at which the functionalized nanotube interacts with alkali metal atoms were determined, the process of scanning an arbitrary virtual surface on which the presence of these atoms is implied by the created nanosystems was simulated to evaluate the sensory interaction between them and the functionalized complexes. Scanning was carried out by modeling the movement of alkali metal atoms along an imaginary straight line drawn perpendicular to the longitudinal axis of the nanotube, successively passing the oxygen atoms of the nitro group at a distance determined at the previous stage of research (Table 3). This trajectory is shown by the dashed line in Fig. (8).
As a result of the performed calculations, the potential energy profiles of the processes were constructed, which allowed us to determine the parameters of sensory interactions in the systems. The obtained data are summarized in Table 4. The distance of 2.6 Å corresponds to the position of the metal atoms strictly in the middle between the oxygen atoms of the nitro group.
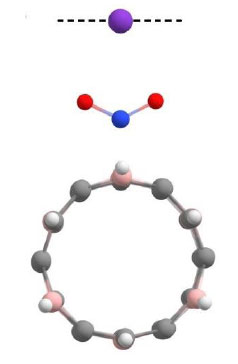
Scanning trajectory of a virtual surface containing a potassium atom (K).
|
Type of Nanotubes |
Adsorption Center |
r, Å | Es-int, eV | Egap, eV | Charge |
|---|---|---|---|---|---|
| Li | |||||
| BC | (a) carbon atom | 2.6 | -3.18 | 0.66 | 0.58 |
| (b) boron atom | 2.6 | -3.89 | 0.95 | 0.54 | |
| BC3 | (a) carbon atom | 2.6 | -3.63 | 0.71 | 0.56 |
| (b) boron atom | 2.6 | -4.33 | 0.7 | 0.53 | |
| BC5 | (a) boron atom | 2.6 | -4.33 | 0.86 | 0.54 |
| Na | |||||
| BC | (a) carbon atom | 2.6 | -2.78 | 0.67 | 0.66 |
| (b) boron atom | 2.6 | -3.35 | 0.95 | 0.62 | |
| BC3 | (a) carbon atom | 2.6 | -3.12 | 0.74 | 0.64 |
| (b) boron atom | 2.6 | -3.76 | 0.7 | 0.6 | |
| BC5 | (b) boron atom | 2.6 | -3.79 | 0.87 | 0.62 |
| K | |||||
| BC | (a) carbon atom | 2.6 | -2.82 | 0.66 | 0.76 |
| (b) boron atom | 2.6 | -3.29 | 0.93 | 0.73 | |
| BC3 | (a) carbon atom | 2.6 | -3.16 | 0.71 | 0.76 |
| (b) boron atom | 2.6 | -3.64 | 0.7 | 0.72 | |
| BC5 | (b) boron atom | 2.6 | -3.71 | 0.88 | 0.74 |
The analysis of the forbidden gap showed that all the systems studied in this work have semiconducting properties.
CONCLUSION
The results of the study of the mechanisms of sensor interaction between alkali metal atoms and boron-carbon nanotubes functionalized with a nitro group containing different amounts of impurity boron atoms replacing carbon atoms of the carbon nanotube surface allow us to conclude that the obtained systems are able to register the presence of selected metal atoms (Li, Na, K). The energetically favorable and preferable location of the nitro group for this process is above the surface boron atom for all boron-carbon nanotubes considered. The conductivity of such systems changes upon interaction with alkali metal atoms, which makes it possible to register their presence. Such systems can act as sensitive elements of sensor devices and detect not only trace amounts of alkali metals, but also other types of contaminants, such as toxic gases and harmful chemicals. The most energetically favorable position for modifying the nanosystems with a functional nitro group is above the boron atom on the nanotube surface. In addition, the increase in impurity boron atoms in the system leads to a decrease in the sorption and sensing interaction energy. More specifically, the addition of a nitro group to nanotubes with a lower concentration of substituting boron atoms is more effective to boron atoms, which can be explained by the greater electronegativity of boron compared to carbon. In the case of a maximum concentration of boron atoms, attachment occurs to a carbon atom, which can be explained by the appearance of charge inhomogeneity on the surface of the nanotube. If we compare this with pure carbon nanotubes [36], the introduction of replacement boron atoms leads to increased interaction with the functional group: the distance changes from 2.2 to 1.6 A. Sensory detection also becomes more likely due to the appearance of greater charge heterogeneity. Thus, it can be concluded that the incorporation of boron atoms even into a carbon nanotube functionalized with a nitro group makes it possible to improve its sensory properties. This is realized due to the fact that charge redistribution occurs in the nanotube, as indicated in the tables. In turn, this electronic heterogeneity leads to increased sensitivity of oxygen atoms included in the nitro group to the detection of elements (for example, metals) due to the electron deficiency resulting from the transfer of electron density from the functional group to the surface of the nanotube, which is usually not observed for pure carbon nanotubes. That is, carbon nanotubes modified with a nitro group with substituted boron atoms can act as effective elements of sensor devices for detecting various substances.
AUTHORS’ CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: Data analysis Or interpretation: I.V.Z; Investigation: E.S.D; Visualization: N.P.B.; Data Curation: S.V.B.; Writing The Paper: P.A.Z.; Methodology: MG. All authors reviewed the results and approved the final version of the manuscript.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, Russia (subject “FZUU-2023-0001”).
ACKNOWLEDGEMENTS
Declared none.


