All published articles of this journal are available on ScienceDirect.
Radioiodine Sorption on AgCl-modified Bentonite and its Stability in different Environments
Abstract
Introduction
This study investigated the stability and radioiodine (I −) sorption properties of AgCl-modified bentonite sorbents under conditions simulating engineered safety barriers (concrete-bentonite contact) in a deep geological radioactive waste disposal facility (GDF).
Methods
Synthetic groundwater from the «Yeniseisky» site was filtered through Portland Cement Concrete (PCC) and High Alumina Concrete (HAC) samples. Filtrate pH/Eh were measured, and chemical composition was analyzed via ICP-MS. AgCl-bentonite sorbents were synthesized using two methods (AgCl HMTA and AgCl HYD). Stability was assessed by monitoring Ag dissolution in filtrates using Volhard’s method. Iodide sorption was evaluated using natural and modified bentonite.
Results
PCC filtrate showed higher pH (12.43) and lower Eh (−74 mV) than HAC filtrate (pH 11.10, Eh +6 mV). PCC contained elevated Ca2+, while HAC contained trace phosphorus. No Ag dissolution occurred from sorbents in filtrates, confirming stability. AgCl-bentonite exhibited significantly higher I − sorption (Kd = 373±87 – 1070±230 mL·g−1) than natural bentonite (K d = 64±28 mL·g −1), with rapid equilibrium (1 hour).
Discussion
The results demonstrate that AgCl-modified bentonite retains high stability and exceptional I −sorption capacity even in alkaline, concrete-impacted environments relevant to GDFs. The absence of Ag dissolution underscores its suitability for long-term containment. However, phosphorus in HAC filtrates may pose a risk of Ag3 PO 4 formation, warranting further study.
1. INTRODUCTION
Nuclear power plants offer significant benefits, including high energy intensity, potential fissile material recovery for reuse, and no carbon dioxide emissions resulting from fossil fuel combustion (e.g., natural gas, coal) used in thermal power plants to generate the majority of global electricity [1, 2]. However, they also present critical technological challenges, particularly regarding the management of generated radioactive waste (RW), specifically high-level waste (HLW) and spent nuclear fuel (SNF). Deep geological isolation in stable host rocks is the most effective method for managing HLW and SNF [2].
To prevent the migration of radionuclides, which pose a significant threat to the biosphere, until their activity drops to acceptable limits, a reliable engineered barrier system (EBS) must be implemented in the deep geological disposal facility for radioactive waste (GDF) [3]. Several leading concepts for radioactive waste isolation in deep geological formations, such as the Swedish KBS-3 and the French Cigéo Project, consider utilizing engineered safety barriers (ESB) comprised of materials like concrete and bentonite in close contact [4, 5]. In Russia, the «Yeniseisky» site (Krasnoyarsk region) has been selected as a site for the GDF. The EBS involves the use of concrete and bentonite in direct contact [6]. Bentonite is currently the most widely accepted material for use as a reliable buffer in the near zone of the GDF due to its high specific surface area, favorable sorption properties for various radionuclides present in isolated radioactive waste [7-9], and the stability of its properties under elevated temperatures [10, 11], chemically aggressive environments [12-17], and low hydraulic permeability attributed to its swelling ability [7].
It is well-known that under the conditions of a GDF, groundwater permeates from the host rock through the concrete, which leads to an increase in its pH due to the dissolution of a number of cement phases [18]. Negative for the key properties of bentonite in the isolation of radioactive waste are pH values of the order of 13 and higher [19, 20], which corresponds to the pH value for fresh cement mortar, and at pH 12–13, this phenomenon is expressed to a much lesser extent. However, an increase in temperature to 80°C and above, which can be caused by heat release from containers with HLW and SNF, intensifies the dissolution of bentonite comparable to the effect of more alkaline solutions [21].
It is worth noting that 235U decay products, such as 99Тс and 129I, are found in waste as anionic species and exhibit substantial mobility in natural bentonite buffer systems [22, 23]. Consequently, the reliable isolation of these products is challenging, particularly for 129I [24] due to its propensity to enter food chains and its long half-life (1.7×107 years). To address this issue, numerous studies have investigated the sorption of iodine anions using various inorganic materials containing iron, copper, sulfur, bismuth, and silver [25-27], as well as layered double hydroxides, metal oxides, silica aerogel, silica gel, organoclays, and activated charcoal [28-34].
A promising approach for immobilizing iodide anions, the most stable and mobile anionic form of radioiodine in the environment [24, 35], involves employing silver-containing sorbents. Examples include materials obtained by coating bentonite particles with metallic Ag or AgCl bentonite, which exhibit enhanced sorption rates and efficiencies for 129I due to their increased specific surface area [36, 37]. Despite the relatively high cost of silver compounds, their usage can be economically justified by incorporating small amounts into compacted natural bentonite clay buffer systems. This approach may reduce the long-term costs of isolating radioactive waste in geological disposal facilities (GDF) by minimizing the need for localizing the impacts of radioiodine compound infiltration on the biosphere.
Considering the transformations that bentonite undergoes in highly alkaline environments, such as those found in GDFs, the performance of silver-containing bentonite sorbents may be influenced by factors such as groundwater filtration from concrete structures in contact with the buffer. Key quality indicators of these sorbents include stable bonds between Ag or AgCl and the base material, as well as high specific surface areas.
The aim of this work is to design a laboratory experimental setup for determining the characteristics of filtrates formed as a result of the impact of a synthetic groundwater solution of a GDF on concrete materials, assess the reliability of AgCl fixation on bentonite, and study the I– sorption properties on modified clay in experimentally obtained aqueous media.
2. MATERIALS AND METHODS
2.1. Materials
The synthetic groundwater solution used in this filtration experiment was prepared based on works [12, 38] and consisted of NaHCO3 (63.88 mg×L–1), MgSO4 (59.41 mg×L–1), CaCl2 (135.08 mg×L–1), and KCl (8.58 mg×L–1). The pH was adjusted to 7 using concentrated HCl solution.
Portland Cement Concrete (PCC) samples were made using CEM I 52.5N (according to Russian standard GOST 31108–202 [39]) binder, quartz sand, and water. High Alumina Concrete (HAC) samples, with a less alkaline reaction due to lower tricalcium silicate and lime content [40-45], were made using calcium aluminate cement of type GTs-35 50 (according to Russian standard GOST 969-2019 [43]), quartz sand, plasticizer C-3, and water.
Two deposition methods were employed: Ag HMTA and Ag HYD [25]. The first method involved saturation of bentonite with a solution of [Ag(NH3)2]OH and a fivefold stoichiometric excess of hexamethylenetetraamine (HMTA) for conversion into the metallic form, followed by reduction at 90°C. The second method consisted of impregnating bentonite with a silver nitrate solution, drying at 90°C for 12 hours, reducing the silver with hydrazonium sulfate in an ammonia medium, and then repeating the drying process at 105°C for another 12 hours.
AgCl-containing sorbents AgCl HMTA and AgCl HYD were obtained by oxidizing the pre-applied metallic Ag on bentonite (10th Khutor deposit, Republic of Khakassia, Russian Federation [44, 45]) surfaces using Ag HMTA and Ag HYD methods, respectively, with at least a twofold stoichiometric excess of iron (III) chloride solution (0.11 M for 0.5-3.0% wt. Ag and 0.28 M for 7.0% wt. Ag).
2.2. Methods
Chemical analysis of the concrete samples was performed using an Axios mAX X-ray spectrometer (PANnalytical, Almelo, the Netherlands). The results showed that SiO2 and CaO were the predominant components in PCC (70.31 and 18.09 wt%, respectively), while SiO2 and Al2O3 were predominant in HAC (67.22 and 16.06 wt%, respectively). HAC also had a 3 times lower CaO content (5.41% wt.) and a lower content of potassium oxide (0.03 and 0.16% wt. for HAC and PCC, respectively), and did not contain SO3 (present in PCC at 0.87% wt.).
The pH of the obtained filtrate solutions was determined using an “Anion 4100” laboratory conductometer-ionomer (Russian Federation), equipped with a combined electrode with a ceramic electrolytic key ESK-10307, calibrated according to standard titers for pH 6.86, 9.18, 12.43. The redox potential of the medium Eh was determined using an EPV-1-100 platinum electrode and an EVL-1M3.1 silver chloride reference electrode calibrated with a Zobell’s solution containing 0.528 g×L–1 K4[Fe(CN)6]·6H2O and 0.412 g×L–1 K3[Fe(CN)6]·H2O in a buffer solution for pH = 7. Analysis of the chemical composition of the filtrates was carried out on an inductively coupled plasma mass spectrometer (ICP-MS) Thermo Scientific iCAP-Qc (USA) equipped with a quadrupole mass analyzer.
The stability of silver fixation on bentonite was assessed using filtrates from PCC and HAC samples obtained from an experimental setup with a solid-to-liquid ratio (S/L) of 1:10 on a vibration stand for one month, followed by centrifugation at 8000 rpm for 10 minutes. Duplicate samples were taken for an additional analysis of AgCl fixation over one year.
The mass fraction of dissolved silver relative to the deposited amount on bentonite in the sample supernatant was determined by titration with a 0.01 M NH4SCN solution using Volhard's method. Based on the titrant concentration and volume per titration portion, the detection limits of dissolved silver were calculated. The absence of silver ions within the detection limits and the stability of the sorbents to the studied media were confirmed by the appearance of the indicator color following the addition of the first titrant portion.
The sorption of microquantities of iodine-131 in the form of I– by natural bentonite and AgCl HMTA and AgCl HYD samples from NRM and NRM PCC with an initial radioiodine activity of 2 103 Bq×mL–1 at S/L = 1:100 (0.5 g of sorbent per 50 mL of liquid phase) and room temperature was studied. The test tubes containing suspensions of studied sorbents in aqueous media were kept on an orbital shaker at a rotation frequency of 170 rpm, after 1; 2; 4; 6 and 24 hours after the start of the experiment the system was centrifuged at 8000 rpm for 10 minutes, a 2 mL sample of the liquid phase was taken and the 131I count rate was determined using a “Progress-gamma” γ-spectrometer (STC “Amplituda”, Russian Federation). The radioiodine sorption yield S, % was calculated using the ratio:
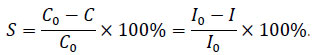 |
(1) |
The interphase distribution coefficient of radioiodine Kd, mL×g-1 was calculated using the formula:
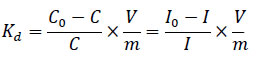 |
(2) |
In the equations (1) and (2), C0 is the initial concentration of iodide ions in the solution (mol×L–1), C is the concentration of iodide ions in the solution at the time of sample collection (mol×L–1), I is the count rate of 131I in the collected sample of the liquid phase (cps), I0 is the count rate of 131I in the sample of the liquid phase collected before the start of the experiment (cps), V is the volume of the liquid phase (mL), m is the mass of the sorbent sample (g).
2.3. Sample Size and Statistical Justification
The sample size in this study was determined based on the following considerations:
2.3.1. Representativeness of the Material
The concrete and bentonite samples used in this study were prepared using standardized procedures to ensure consistency and representativeness. The concrete samples (Portland Cement Concrete and High Alumina Concrete) were designed to mimic the materials proposed for use in engineered barriers in deep geological repositories. The bentonite samples, modified with silver chloride (AgCl), were prepared using natural bentonite from the 10th Khutor deposit, which is representative of the buffer material intended for use in the near-field of a GDF. Given the homogeneity of the materials and the controlled conditions under which the samples were prepared, a smaller number of samples was deemed sufficient to capture the material's properties.
2.3.2. Experimental Constraints
The study involved extensive experimental procedures, including the filtration of synthetic groundwater through concrete samples, chemical analysis of filtrates, and sorption experiments with radioiodine. These methods require significant time and resources, which limit the number of samples that can be processed. However, the samples were carefully selected to ensure they were representative of the material's behavior under the studied conditions.
2.3.3. Previous Studies and Standards
The sample size was also informed by previous studies on bentonite-concrete interactions and sorption properties of AgCl-modified bentonite, where similar sample sizes were used to achieve reliable results [7, 25, 36, 46]. Additionally, the sample preparation and testing procedures followed established standards and guidelines, ensuring the reproducibility and reliability of the results.
The sample size in this study was determined based on material representativeness, experimental constraints, and alignment with prior research. Standardized preparation methods ensured homogeneity of concrete and bentonite samples, reducing variability. Due to resource-intensive procedures (e.g., synthetic groundwater filtration, ICP-MS analysis, radioiodine sorption experiments), a smaller sample size was pragmatically selected while maintaining reproducibility through triplicate measurements for key parameters (pH, Eh, sorption efficiency). These replicates allowed calculation of standard deviations and confidence intervals (±15%), confirming consistency. The sample size aligns with previous studies on bentonite-concrete interactions and AgCl-modified sorbents, where similar scales achieved statistically robust results. Detection limits for dissolved silver (0.3-4.1%) and sorption experiments (Kd values) fell within expected ranges, consistent with literature data, further supporting adequacy. While a formal power analysis was not conducted due to the exploratory nature of the work, the close agreement between experimental results, error margins (1–15%), and published findings indicates that the sample size sufficed to capture material behavior and validate hypotheses under the studied conditions.
To ensure the reliability and statistical significance of the results, the following steps were taken:
1) Replication of Measurements: Each experimental measurement (e.g., pH, Eh, chemical composition of filtrates, and sorption experiments) was performed in triplicate to account for variability and ensure reproducibility. The average values and standard deviations were calculated to assess the consistency of the results.
2) Error Analysis: The relative error between experimental and simulated results was calculated for key parameters such as pH, Eh, and sorption efficiency. The small relative errors (ranging from 1% to 15%) indicate that the sample size was adequate to achieve reliable results.
3) Comparison with Literature Data: The results obtained in this study were compared with literature data [7, 25, 37, 46], and the close agreement further supports the adequacy of the sample size. The sorption properties of AgCl-modified bentonite were consistent with previously reported values, indicating that the sample size was sufficient to capture the material's behavior.
4) Power Analysis: Although a formal power analysis was not conducted due to the exploratory nature of the study, the consistency of the results across multiple measurements and the agreement with literature data suggest that the sample size was appropriate for the objectives of this study.
In conclusion, the sample size was selected based on the representativeness of the material, experimental constraints, and adherence to established standards. The replication of measurements, error analysis, and comparison with literature data provide statistical justification for the adequacy of the sample size in achieving reliable and statistically significant results.
The study acknowledges several limitations. Experimental conditions were controlled and static, potentially oversimplifying real-world complexities such as long-term temperature fluctuations, mechanical stress, or radiation exposure in geological disposal facilities (GDFs). Short-term testing (1–28 days) may not capture long-term AgCl dissolution or secondary mineral formation (e.g., silver phosphate in phosphorus-containing environments), which could affect sorbent performance over millennia.
Two concrete types (PCC and HAC) were tested, limiting generalizability to other cementitious materials. Methodological constraints include batch experiments that do not simulate dynamic groundwater flow and a limited sample size, which, while justified by prior studies and resource constraints, may reduce statistical robustness. Detection limits for dissolved silver (~0.3–4.1%) could miss trace leaching, and assumptions about anoxic conditions or homogeneous groundwater composition might not reflect heterogeneous field scenarios.
Scalability of synthesis methods (e.g., hydrazine use) and regulatory hurdles for silver-containing materials in GDFs also remain unresolved. These limitations highlight the need for extended testing under realistic conditions, including dynamic flow, multi-radionuclide systems, and long-term geochemical modeling to validate the sorbents’ viability for GDF applications.
3. RESULTS AND DISCUSSION
To obtain filtrates of a synthetic groundwater solution, a laboratory setup was constructed. In this experimental setup (Fig. 1), a synthetic groundwater solution of the crystalline rocks of the Yeniseisky site was passed under a pressure of 1 MPa through the filtration cells containing concrete samples with a diameter of 47 mm and a height of 31 mm [47]. A preliminary purge of all units of the setup and injection of the necessary pressure in the system was carried out using a cylinder with nitrogen of high purity (99.9999%). The use of compressed air, unlike nitrogen, will lead to the inevitable passivation of the mineral surfaces of concrete due to the interaction of atmospheric CO2 with portlandite (Ca(OH)2) with the formation of calcite (CaCO3), which will lead to a decrease in the pH of the filtrate and distortion of the experimental results [48].
As a result of the experiment on the constructed setup, filtrates of synthetic groundwater solution of the «Yeniseisky» site were obtained through PCC and HAC samples and their pH and Eh were determined and presented in (Fig. 2a-b) and (Fig. 2c-d) respectively. The resulting graphs show a strong negative correlation of pH vs. Eh. To predict the long-term stability of synthesized sorbents accurately, it is essential to examine their interaction with the aquatic medium at a specific time interval after initiating the experiment. Therefore, to assess the reliability of fixing AgCl on the surface of bentonite, NRM PCC and NRM HAC filtrates sampled after 28 days of the experiment were used, the parameters of which are listed in Table 1 and displayed the most aggressive conditions among the obtained data to justify.
| Filtrate Sample | pH | Eh, mV |
|---|---|---|
| NRM PCC | 12.35 | -55 |
| NRM HAC | 10.97 | 34 |
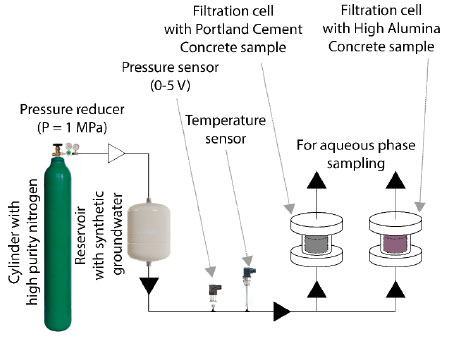
Schematic image of the designed setup.
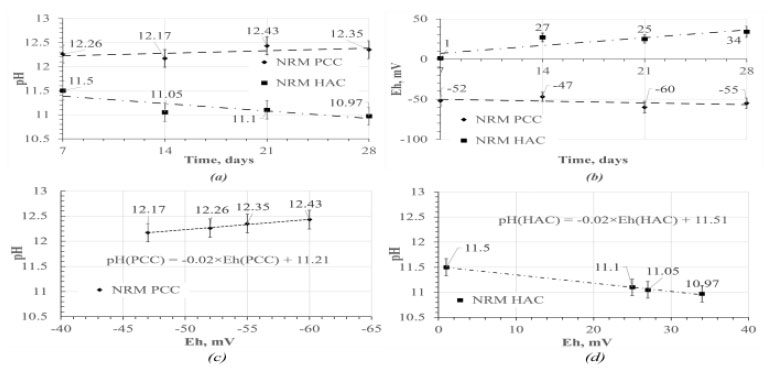
pH (a) and Eh (b) vs. time of the experiment, determined for samples of the studied filtrates; pH vs. Eh obtained for PCC (c) and HAC (d) filtrates.
the selection of the aqueous environment for the assession of AgCl-modified bentonite stability.
On the Pourbaix diagram for AgCl in [49], silver chloride in the obtained pH and Eh ranges (10.97 - 12.35 and –55 - 34 mV, respectively) of the medium in the analyzed filtrates do not undergo redox reactions leading to the formation of soluble forms of Ag, on the basis of which it is possible to make a preliminary prediction about the absence of its dissolution from the surface of sorbents under simulated conditions of hydrochemical interaction with concrete in the GDF. At the same time, the reaction of reduction of AgCl to metallic silver at pH-Eh, obtained from the analysis of concrete filtrate solutions, is possible. This phenomenon can potentially indicate a decrease in the efficiency of the sorbent, since the sorption value of the sorbent with silver in the form of chloride is higher than that of the analogous one with Ag deposited on the surface [36]. The results of the analysis of the synthetic groundwater solution filtrates passed through the PCC and HAC concrete samples' chemical composition by the ICP-MS are presented in Table 2.
Based on the obtained data, the high pH of the PCC filtrate is mainly due to the higher content of Ca(OH)2, the concentration of which is an order of magnitude higher than in the sample of the HAC filtrate. Sulfur-containing anions are absent in both samples, which in the future will have a positive effect on the performance of the sorbent due to the impossibility of forming Ag2S less soluble than silver iodide and chloride (Ksp(AgI) = 8.52×10-17, Ksp(AgCl) = 1.77×10-10, Ksp(Ag2S) = 6.30×10-50) [36, 49]. However, in the High Alumina Concrete filtrate medium, a formation of less soluble silver phosphate is possible due to the presence of small amounts of phosphorus in the sample and a lower solubility product (Ksp(Ag3PO4) = 1.46×10-21) [50, 51], which in turn requires further study.
In the course of the experiment to assess the reliability of fixing silver on the surface of bentonite in the studied sorbents under the influence of model conditions of hydrochemical contact with concrete in the GDF, the results presented in Table 3 were obtained.
The obtained experimental data on the quantitative dissolution of silver are below the detection limits established during the titration of a blank sample of the supernatant solution of centrifuged suspensions of natural bentonite in the media used by the Volhard’s method, subject to manipulations similar to those for silver-containing sorbents, which indicates a reliable fixation of AgCl on the modified bentonite at the impact of dissolved products of cement hydration in the synthetic groundwater during filtration through concrete in the GDF. For comparison, a sorbent based on natural bentonite from the Zyryanskoe deposit (Kurgan region, Russian Federation) with applied AgCl in an amount of 7% shows the dissolution of silver in 1–3 M HNO3 solutions of about 41%, which indicates the stability of the obtained sorbents to the maximum possible aggressive media, the scenario of which occurrence in GDF is impossible [36].
| Component of Solution | Concentration, 10-4 mol×L–1 | ||
|---|---|---|---|
| NRM | NRM PCC | NRM HAC | |
| Na | 12.60 | 11.41 | 14.30 |
| K | 1.70 | 2.92 | 1.78 |
| Ca | 17.95 | 154.31 | 19.87 |
| Al | 3.58 | 2.97 | 40.25 |
| Fe | 4.52×10-2 | 1.66×10-1 | 2.31×10-2 |
| Mg | 7.28 | 2.65×10-3 | 1.48×10-2 |
| Si | 3.78 | 5.90 | 23.51 |
| S | <LOD | < LOD* | < LOD |
| P | 6.89×10-2 | < LOD | 1.14×10-1 |
| Type of Sorbent | Amount of Dissolved Silver ωAg, % | |
|---|---|---|
| NRM PCC | NRM HAC | |
| AgCl 0.5% HMTA | < 4.1±1.7 | < 3.7±3.2 |
| AgCl 0.5% HYD | ||
| AgCl 3.0% HMTA | < 0.7±0.3 | < 0.6±0.5 |
| AgCl 3.0% HYD | ||
| AgCl 7.0% HMTA | < 0.3±0.1 | < 0.3±0.2 |
| AgCl 7.0% HYD | ||
| Type of Sorbent | NRM | NRM PCC | ||
|---|---|---|---|---|
| S, % | Kd, mL×g-1 | S, % | Kd, mL×g-1 | |
| Natural bentonite | 39±12 | 64±28 | 34±12 | 52±26 |
| AgCl 0.5% HMTA | 88±13 | 720±150 | 90±14 | 890±200 |
| AgCl 0.5% HYD | 87±13 | 640±140 | 85±13 | 560±120 |
| AgCl 3.0% HMTA | 79±13 | 373±87 | 90±14 | 880±190 |
| AgCl 3.0% HYD | 89±13 | 850±170 | 86±13 | 610±130 |
| AgCl 7.0% HMTA | 84±13 | 540±120 | 89±13 | 830±170 |
| AgCl 7.0% HYD | 91±14 | 1070±230 | 86±13 | 620±130 |
Previously, high sorption properties of bentonite with silver chloride applied to the surface with respect to iodide anion in distilled water were established (the equilibrium sorption yield was 99±14%) [45]. In this article, the sorption of iodide ions by bentonite samples with silver chloride AgCl HMTA and AgCl HYD from NRM solution and the filtrate of synthetic groundwater through PCC samples was investigated. NRM PCC was selected for sorption studies as the most negative scenario in GDF due to its high alkalinity and the greatest prevalence of materials based on ordinary Portland cement in concepts of geological disposal of radioactive waste [5]. As a result of the study of the kinetics of sorption of iodide ions, it was revealed that equilibrium occurs 1 hour after the start of testing for all studied samples, regardless of the medium used.
The calculated equilibrium sorption yields and the interphase distribution coefficients are given in Table 4.
The obtained data indicate low sorption properties of the natural bentonite from the 10th Khutor deposit in relation to iodide ions, under the studied conditions, the equilibrium iodine sorption yield does not exceed 40%, and the interphase distribution coefficient is 70 mL×g–1. Application of silver chloride already in the amount of 0.5% of the clay sample weight leads to an increase in the interphase distribution coefficient of iodine by more than an order of magnitude (Kd = 373 ± 87 - 1070 ± 230 mL×g-1), the values of the sorption yield (S = 79 ± 13 - 91 ± 14%) indicate almost complete removal of radioactive iodide ions from the liquid phase. It can be noted that, although all the obtained values of Kd and S for iodine on the samples of AgCl-modified bentonite are close, with equal AgCl content in the same media, the AgCl HYD samples provide slightly higher sorption yields and interphase distribution coefficients of iodine than the AgCl HMTA samples. It was established that, in comparison with sorption experiments conducted in distilled water [46], AgCl-modified bentonite in NRM and NRM PCC solutions shows a practically identical insignificant decrease in S and Kd, the differences in which do not go beyond the confidence intervals. Thus, it can be concluded that the efficiency of iodide ion removal by the studied sorbents is greatly influenced by the presence of foreign ions, while a highly alkaline environment caused by the interaction of groundwater with the PCC will not have a noticeable effect.
CONCLUSION
In this study, an experimental setup was designed and constructed to investigate the characteristics of synthetic groundwater filtrates from the «Yeniseisky» site of the Nizhne-Kansky crystalline rock massif, which is a proposed location for a GDF for radioactive waste in Russia. The study focused on the stability and sorption properties of silver chloride (AgCl)-modified bentonite in the presence of concrete ESB, particularly under conditions where bentonite comes into contact with concrete mixtures.
The results demonstrated that the synthetic groundwater filtrates passing through PCC and HAC samples exhibited distinct pH and redox potential (Eh) values. The PCC filtrate had a higher pH (12.35) and lower Eh (-55 mV) compared to the HAC filtrate (pH 10.97, Eh 34 mV). These differences were attributed to the higher calcium hydroxide (Ca(OH)2) content in the PCC filtrate, which is consistent with the chemical composition of the concrete samples. The presence of phosphorus in the HAC filtrate, albeit in small amounts, suggests the potential formation of less soluble silver phosphate (Ag3PO4), which could impact the performance of AgCl-modified bentonite sorbents. This finding warrants further investigation to understand the long-term implications for the stability of silver-containing sorbents in GDF environments.
The study also confirmed the high stability of AgCl-modified bentonite sorbents in aqueous media simulating the hydrochemical conditions of a GDF. Dissolution of AgCl was not observed in the filtrates, regardless of the method used to apply the silver-containing compounds (Ag HMTA or Ag HYD). This indicates that the sorbents are highly resistant to the alkaline conditions created by the interaction of groundwater with concrete, which is a critical factor for their application in GDFs.
Furthermore, the sorption experiments revealed that AgCl-modified bentonite exhibited excellent sorption properties for iodide ions (I−), with sorption yields exceeding 85% in both synthetic groundwater (NRM) and PCC filtrate. The interphase distribution coefficients (Kd) for radioiodine were significantly higher for AgCl-modified bentonite compared to natural bentonite, demonstrating the effectiveness of silver chloride in enhancing the sorption capacity of bentonite for anionic radionuclides. The sorption equilibrium was achieved within one hour, indicating rapid and efficient uptake of iodide ions by the modified sorbents.
The findings of this study have important implications for the design and implementation of EBS in GDFs. The high stability and sorption efficiency of AgCl-modified bentonite suggest that it is a promising material for use in the buffer layer of a GDF, particularly in scenarios where bentonite comes into contact with concrete ESB. The ability of these sorbents to effectively capture radioiodine, even in the presence of highly alkaline conditions, supports their potential inclusion in the Russian deep isolation project for radioactive waste in crystalline host rock at the «Yeniseisky» site.
However, it is important to acknowledge certain limitations of this study. The experiments were conducted under controlled laboratory conditions, which may not fully replicate the complex and variable environmental conditions in a deep geological repository. Factors such as long-term temperature fluctuations, mechanical stress, and the presence of other chemical species in groundwater could influence the performance of the sorbents.
Future research should focus on:
- Conducting long-term experiments to assess the stability and sorption properties of AgCl-modified bentonite under more realistic GDF conditions, including elevated temperatures and mechanical stress.
- Investigating the potential formation of silver phosphate (Ag3PO4) in the presence of phosphorus and its impact on the sorption efficiency of AgCl-modified bentonite.
- Developing advanced modeling techniques to predict the long-term behavior of AgCl-modified bentonite in GDF environments, taking into account the kinetics of sorption and desorption processes.
In conclusion, this study provides valuable insights into the stability and sorption properties of AgCl-modified bentonite in the context of deep geological disposal of radioactive waste. The results demonstrate the potential of these materials to enhance the performance of engineered barrier systems, particularly in scenarios where bentonite comes into contact with concrete. By addressing the limitations and expanding the scope of future research, it will be possible to further optimize the design of EBS for the safe and effective isolation of radioactive waste in GDFs.
AUTHORS' CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: A.P.: Data collection; V.K.: Validation; E.T., P.K.: Draft manuscript; All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| EBS | = Engineered Barrier System |
| ESB | = Engineered Safety Barrier |
| GDF | = Geological Disposal Facility |
| GTS | = Grimsel Test Site |
| HAC | = High Alumina Concrete |
| HLW | = High Level Waste |
| HTMA | = Hexamethylenetetramine |
| HYD | = Hydrazine |
| ICP-MS | = Inductively Coupled Plasma Mass Spectrometry |
| ILW | = Intermediate Level Waste |
| L/S | = Liquid-To-Solid Ratio |
| NRM | = Nizhne-Kansky Rock Massif |
| PCC | = Portland Cement Concrete |
| RW | = Radioactive Waste |
| SKB | = Svensk Kärnbränslehantering Aktiebolag |
| SNF | = Spent Nuclear Fuel |
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this research are available within the article.
FUNDING
The study of sorbents' stability under conditions of contact with concrete in a deep geological disposal facility was supported by Russian Science Foundation project #22-29-0067, Developing of silver-containing bentonite-based sorbent for uptake of anionic forms of radioactive iodine in radioactive waste repositories.
ACKNOWLEDGEMENTS
Declared none.


